Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer
- PMID: 23402814
- PMCID: PMC3665739
- DOI: 10.1016/j.canlet.2013.01.054
Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer
Abstract
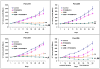
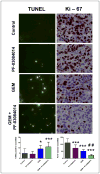
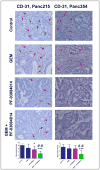
Pancreatic ductal adenocarcinoma (PDA) remains a lethal human malignancy with historically limited success in treatment. The role of aberrant Notch signaling, which requires the constitutive activation of γ-secretase, in the initiation and progression of PDA is well defined and inhibitors of this pathway are currently in clinical trials. Here we investigated the in vivo therapeutic effect of PF-03084014, a selective γ-secretase inhibitor, alone and in combination with gemcitabine in pancreatic cancer xenografts. PF-03084014 treatment inhibited the cleavage of nuclear Notch 1 intracellular domain and Notch targets Hes-1 and Hey-1. Gemcitabine treatment showed good response but not capable of inducing tumor regressions and targeting the tumor-resident cancer stem cells (CD24(+)CD44(+) and ALDH(+) tumor cells). A combination of PF-03084014 and gemcitabine treatment resulted tumor regression in 3 of 4 subcutaneously implanted xenograft models. PF-03084014, and in combination with gemcitabine reduced putative cancer stem cells, indicating that PF-03084014 target the especially dangerous and resilient cancer stem cells within pancreatic tumors. Tumor re-growth curves plotted after drug treatments demonstrated that the effect of the combination therapy was sustainable than that of gemcitabine. Notably, in a highly aggressive orthotopic model, PF-03084014 and gemcitabine combination was effective in inducing apoptosis, inhibition of tumor cell proliferation and angiogenesis, resulting in the attenuation of primary tumor growth as well as controlling metastatic dissemination, compared to gemcitabine treatment. In summary, our preclinical data suggest that PF-03084014 has greater anti-tumor activity in combination with gemcitabine in PDA and provides rationale for further investigation of this combination in PDA.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
None
Figures





References
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. - PubMed
-
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

