Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties
- PMID: 23402889
- PMCID: PMC3654025
- DOI: 10.1016/j.jdent.2013.02.001
Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties
Abstract
Objectives: The objective of this study was to investigate the effects of dentine primer containing dual antibacterial agents, namely, 12-methacryloyloxydodecylpyridinium bromide (MDPB) and nanoparticles of silver (NAg), on dentine bond strength, dental plaque microcosm biofilm response, and fibroblast cytotoxicity for the first time.
Methods: Scotchbond Multi-Purpose (SBMP) was used as the parent bonding agent. Four primers were tested: SBMP primer control (referred to as "P"), P+5% MDPB, P+0.05% NAg, and P+5% MDPB+0.05% NAg. Dentine shear bond strengths were measured using extracted human teeth. Biofilms from the mixed saliva of 10 donors were cultured to investigate metabolic activity, colony-forming units (CFU), and lactic acid production. Human fibroblast cytotoxicity of the four primers was tested in vitro.
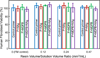
Results: Incorporating MDPB and NAg into primer did not reduce dentine bond strength compared to control (p>0.1). SEM revealed well-bonded adhesive-dentine interfaces with numerous resin tags. MDPB or NAg each greatly reduced biofilm viability and acid production, compared to control. Dual agents MDPB+NAg had a much stronger effect than either agent alone (p<0.05), increasing inhibition zone size and reducing metabolic activity, CFU and lactic acid by an order of magnitude, compared to control. There was no difference in cytotoxicity between commercial control and antibacterial primers (p>0.1).
Conclusions: The method of using dual agents MDPB+NAg in the primer yielded potent antibacterial properties. Hence, this method may be promising to combat residual bacteria in tooth cavity and invading bacteria at the margins. The dual agents MDPB+NAg may have wide applicability to other adhesives, composites, sealants and cements to inhibit biofilms and caries.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures





References
-
- Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. American Journal of Dentistry. 2009;22:3–8. - PubMed
-
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital and Health Statistics. 2007;11:1–92. - PubMed
-
- Deng DM, ten Cate JM. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Research. 2004;38:54–61. - PubMed
-
- Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Research. 2007;41:467–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

