Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-β via their tumor-tropic effect in cellular and xenograft mouse models
- PMID: 23403306
- PMCID: PMC5528496
- DOI: 10.1016/j.molonc.2013.01.004
Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-β via their tumor-tropic effect in cellular and xenograft mouse models
Abstract
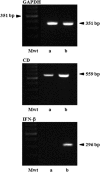
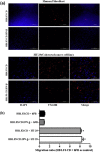
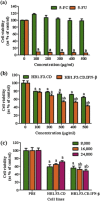
Genetically engineered stem cells (GESTECs) exhibit a potent therapeutic efficacy via their strong tumor tropism toward cancer cells. In this study, we introduced the human parental neural stem cells, HB1.F3, with the human interferon beta (IFN-β) gene which is a typical cytokine gene that has an antitumor effect and the cytosine deaminase (CD) gene from Escherichia coli (E. coli) that could convert the non-toxic prodrug, 5-fluorocytosine (5-FC), to a toxic metabolite, 5-fluorouracil (5-FU). Two types of stem cells expressing the CD gene (HB1.F3.CD cells) and both the CD and human IFN-β genes (HB1.F3.CD.IFN-β) were generated. The present study was performed to examine the migratory and therapeutic effects of these GESTECs against the colorectal cancer cell line, HT-29. When co-cultured with colorectal cancer cells in the presence of 5-FC, HB1.F3.CD and HB1.F3.CD.IFN-β cells exhibited the cytotoxicity on HT-29 cells via the bystander effect. In particular, HB1.F3.CD.IFN-β cells showed the synergistic cytotoxic activity of 5-FU and IFN-β. We also confirmed the migration ability of HB1.F3.CD and HB1.F3.CD.IFN-β cells toward HT-29 cells by a modified migration assay in vitro, where chemoattractant factors secreted by HT-29 cells attracted the GESTECs. In a xenograft mouse model, the volume of tumor mass was decreased up to 56% in HB1.F3.CD injected mice while the tumor mass was greatly inhibited about 76% in HB1.F3.CD.IFN-β injected mice. The therapeutic treatment by these GESTECs is a novel strategy where the combination of the migration capacity of stem cells as a vector for therapeutic genes towards colorectal cancer and a synergistic antitumor effect of CD and IFN-β genes can selectively target this type of cancer.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Aboody, K.S. , Bush, R.A. , Garcia, E. , Metz, M.Z. , Najbauer, J. , Justus, K.A. , Phelps, D.A. , Remack, J.S. , Yoon, K.J. , Gillespie, S. , Kim, S.U. , Glackin, C.A. , Potter, P.M. , Danks, M.K. , 2006. Development of a tumor-selective approach to treat metastatic cancer. PLoS One 1, e23 - PMC - PubMed
-
- Aboody, K.S. , Najbauer, J. , Danks, M.K. , 2008. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 15, 739–752. - PubMed
-
- Altaner, C. , 2008. Prodrug cancer gene therapy. Cancer Lett. 270, 191–201. - PubMed
-
- Bird, N.C. , Mangnall, D. , Majeed, A.W. , 2006. Biology of colorectal liver metastases: a review. J. Surg. Oncol. 94, 68–80. - PubMed
-
- Boucher, P.D. , Im, M.M. , Freytag, S.O. , Shewach, D.S. , 2006. A novel mechanism of synergistic cytotoxicity with 5-fluorocytosine and ganciclovir in double suicide gene therapy. Cancer Res. 66, 3230–3237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

