Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia
- PMID: 23403405
- PMCID: PMC3672228
- DOI: 10.1016/j.bone.2013.01.046
Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia
Abstract
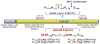
Although recent studies have established that osteocytes function as secretory cells that regulate phosphate metabolism, the biomolecular mechanism(s) underlying these effects remain incompletely defined. However, investigations focusing on the pathogenesis of X-linked hypophosphatemia (XLH), autosomal dominant hypophosphatemic rickets (ADHR), and autosomal recessive hypophosphatemic rickets (ARHR), heritable disorders characterized by abnormal renal phosphate wasting and bone mineralization, have clearly implicated FGF23 as a central factor in osteocytes underlying renal phosphate wasting, documented new molecular pathways regulating FGF23 production, and revealed complementary abnormalities in osteocytes that regulate bone mineralization. The seminal observations leading to these discoveries were the following: 1) mutations in FGF23 cause ADHR by limiting cleavage of the bioactive intact molecule, at a subtilisin-like protein convertase (SPC) site, resulting in increased circulating FGF23 levels and hypophosphatemia; 2) mutations in DMP1 cause ARHR, not only by increasing serum FGF23, albeit by enhanced production and not limited cleavage, but also by limiting production of the active DMP1 component, the C-terminal fragment, resulting in dysregulated production of DKK1 and β-catenin, which contributes to impaired bone mineralization; and 3) mutations in PHEX cause XLH both by altering FGF23 proteolysis and production and causing dysregulated production of DKK1 and β-catenin, similar to abnormalities in ADHR and ARHR, but secondary to different central pathophysiological events. These discoveries indicate that ADHR, XLH, and ARHR represent three related heritable hypophosphatemic diseases that arise from mutations in, or dysregulation of, a single common gene product, FGF23 and, in ARHR and XLH, complimentary DMP1 and PHEX directed events that contribute to abnormal bone mineralization.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
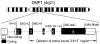

References
-
- Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;6:281–290. - PubMed
-
- Bianchine JW, Stambler AA, Harrison HE. Familial hypophosphatemic rickets showing autosomal dominant inheritance. Birth Defects Orig Artic Ser. 1971;7:287–295. - PubMed
-
- Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82:674–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

