Single-dose safety, tolerability, and pharmacokinetics of the antibiotic GSK1322322, a novel peptide deformylase inhibitor
- PMID: 23403431
- PMCID: PMC3632897
- DOI: 10.1128/AAC.01779-12
Single-dose safety, tolerability, and pharmacokinetics of the antibiotic GSK1322322, a novel peptide deformylase inhibitor
Abstract
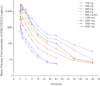
GSK1322322 is a potent inhibitor of peptide deformylase, an essential bacterial enzyme required for protein maturation. GSK1322322 is active against community-acquired skin and respiratory tract pathogens, including methicillin-resistant Staphylococcus aureus, multidrug-resistant Streptococcus pneumoniae, and atypical pathogens. This phase I, randomized, double-blind, placebo-controlled, 2-part, single-dose, dose escalation study (first time in humans) evaluated the safety, tolerability, and pharmacokinetics of GSK1322322 (powder-in-bottle formulation) in healthy volunteers. In part A, dose escalation included GSK1322322 doses of 100, 200, 400, 800, and 1,500 mg under fasting conditions and 800 mg administered with a high-fat meal. In part B, higher doses of GSK1322322 (2,000, 3,000, and 4,000 mg) were evaluated under fasting conditions. Of the 39 volunteers enrolled in the study, 29 and 10 volunteers were treated with GSK1322322 and placebo, respectively. Upon single-dose administration, GSK1322322 was absorbed rapidly, with median times to maximum plasma concentration (T(max)) ranging from 0.5 to 1.0 h. The maximum observed plasma concentration (C(max)) and exposure (area under the concentration-time curve [AUC]) of GSK1322322 were greater than dose proportional between 100 and 1,500 mg and less than dose proportional between 1,500 and 4,000 mg. Administration of the drug with a high-fat meal reduced the rate of absorption (reduced C(max) and delayed T(max)) without affecting the extent of absorption (no effect on AUC). GSK1322322 was generally well tolerated, with all adverse events being mild to moderate in intensity during both parts of the study. The most frequently reported adverse event was headache. Data from this study support further evaluation of GSK1322322.
Figures
References
-
- Schmieder R, Edwards R. 2012. Insights into antibiotic resistance through metagenomic approaches. Future Microbiol. 7:73–89 - PubMed
-
- Rehm SJ, Tice A. 2010. Staphylococcus aureus: methicillin-susceptible S. aureus to methicillin-resistant S. aureus and vancomycin-resistant S. aureus. Clin. Infect. Dis. 51(Suppl 2):S176–S182 doi:10.1086/653518 - DOI - PubMed
-
- Song JH, Chung DR. 2010. Respiratory infections due to drug-resistant bacteria. Infect. Dis. Clin. North Am. 24:639–653 - PubMed
-
- Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D. 2010. Sources of novel antibiotics—aside the common roads. Appl. Microbiol. Biotechnol. 88:1261–1267 - PubMed
-
- Lewandowski T, Peters T, Simon N, Kulkarni S. 2010. Potent activity of GSK1322322 a novel peptide deformylase inhibitor in a Haemophilus influenzae and Streptococcus pneumoniae respiratory tract infection model. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 http://www.icaac.org/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical