Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides
- PMID: 23403432
- PMCID: PMC3632893
- DOI: 10.1128/AAC.02588-12
Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides
Abstract
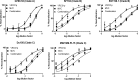
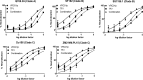
Broadly neutralizing monoclonal antibodies (bnMAbs) may offer powerful tools for HIV-1 preexposure prophylaxis, such as topical microbicides. However, this option is hampered due to expensive MAb biomanufacturing based on mammalian cell culture. To address this issue, we developed a new production system for bnMAb VRC01 in Nicotiana benthamiana plants using a tobamovirus replicon vector. Unlike conventional two-vector-based expression, this system was designed to overexpress full-length IgG1 from a single polypeptide by means of kex2p-like enzyme recognition sites introduced between the heavy and light chains. An enzyme-linked immunosorbent assay (ELISA) revealed that gp120-binding VRC01 IgG1 was maximally accumulated on 5 to 7 days following vector inoculation, yielding ~150 mg of the bnMAb per kg of fresh leaf material. The plant-made VRC01 (VRC01p) was efficiently purified by protein A affinity followed by hydrophobic-interaction chromatography. ELISA, surface plasmon resonance, and an HIV-1 neutralization assay demonstrated that VRC01p has gp120-binding affinity and HIV-1-neutralization capacity virtually identical to the human-cell-produced counterpart. To advance VRC01p's use in topical microbicides, we analyzed combinations of the bnMAb with other microbicide candidates holding distinct antiviral mechanisms in an HIV-1 neutralization assay. VRC01p exhibited clear synergy with the antiviral lectin griffithsin, the CCR5 antagonist maraviroc, and the reverse transcriptase inhibitor tenofovir in multiple CCR5-tropic HIV-1 strains from clades A, B, and C. In summary, VRC01p is amenable to robust, rapid, and large-scale production and may be developed as an active component in combination microbicides with other anti-HIV agents such as antiviral lectins, CCR5 antagonists, and reverse transcriptase inhibitors.
Figures



Similar articles
-
Characterization of VRC01, a potent and broadly neutralizing anti-HIV mAb, produced in transiently and stably transformed tobacco.Plant Biotechnol J. 2014 Apr;12(3):300-11. doi: 10.1111/pbi.12137. Epub 2013 Nov 21. Plant Biotechnol J. 2014. PMID: 24256218 Free PMC article.
-
A novel anti-HIV-1 bispecific bNAb-lectin fusion protein engineered in a plant-based transient expression system.Plant Biotechnol J. 2019 Aug;17(8):1646-1656. doi: 10.1111/pbi.13090. Epub 2019 Mar 12. Plant Biotechnol J. 2019. PMID: 30729651 Free PMC article.
-
Exposure to entry inhibitors alters HIV infectiousness and sensitivity to broadly neutralizing monoclonal antibodies.J Acquir Immune Defic Syndr. 2014 Sep 1;67(1):7-14. doi: 10.1097/QAI.0000000000000223. J Acquir Immune Defic Syndr. 2014. PMID: 24872133
-
HIV Broadly Neutralizing Antibodies: VRC01 and Beyond.Adv Exp Med Biol. 2018;1075:53-72. doi: 10.1007/978-981-13-0484-2_3. Adv Exp Med Biol. 2018. PMID: 30030789 Review.
-
A matrix of structure-based designs yields improved VRC01-class antibodies for HIV-1 therapy and prevention.MAbs. 2021 Jan-Dec;13(1):1946918. doi: 10.1080/19420862.2021.1946918. MAbs. 2021. PMID: 34328065 Free PMC article. Review.
Cited by
-
The potential of plant systems to break the HIV-TB link.Plant Biotechnol J. 2019 Oct;17(10):1868-1891. doi: 10.1111/pbi.13110. Epub 2019 Jul 18. Plant Biotechnol J. 2019. PMID: 30908823 Free PMC article. Review.
-
Plant virus expression vector development: new perspectives.Biomed Res Int. 2014;2014:785382. doi: 10.1155/2014/785382. Epub 2014 Mar 13. Biomed Res Int. 2014. PMID: 24745025 Free PMC article. Review.
-
HRA2pl peptide: a fusion inhibitor for human metapneumovirus produced in tobacco plants by transient transformation.Planta. 2015 Jul;242(1):69-76. doi: 10.1007/s00425-015-2277-5. Epub 2015 Apr 1. Planta. 2015. PMID: 25828350
-
Characterization of VRC01, a potent and broadly neutralizing anti-HIV mAb, produced in transiently and stably transformed tobacco.Plant Biotechnol J. 2014 Apr;12(3):300-11. doi: 10.1111/pbi.12137. Epub 2013 Nov 21. Plant Biotechnol J. 2014. PMID: 24256218 Free PMC article.
-
A novel anti-HIV-1 bispecific bNAb-lectin fusion protein engineered in a plant-based transient expression system.Plant Biotechnol J. 2019 Aug;17(8):1646-1656. doi: 10.1111/pbi.13090. Epub 2019 Mar 12. Plant Biotechnol J. 2019. PMID: 30729651 Free PMC article.
References
-
- 2012. Global report: UNAIDS report on the global AIDS epidemic 2012. Joint United Nations Programme on HIV/AIDS. UNAIDS, Geneva, Switzerland:
-
- Buckheit KW, Buckheit RW., Jr 2012. Factors important to the prioritization and development of successful topical microbicides for HIV-1. Mol. Biol. Int. 2012:781305 doi:10.1155/2012/781305 - DOI - PMC - PubMed
-
- McGowan I. 2010. Microbicides for HIV prevention: reality or hope? Curr. Opin. Infect. Dis. 23:26–31 - PubMed
-
- Van Damme L, Szpir M. 2012. Current status of topical antiretroviral chemoprophylaxis. Curr. Opin. HIV AIDS 7:520–525 - PubMed
-
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

