Bis-(3'-5')-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa
- PMID: 23403434
- PMCID: PMC3632963
- DOI: 10.1128/AAC.02499-12
Bis-(3'-5')-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa
Abstract
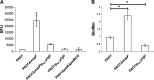
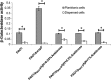
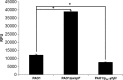
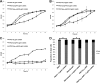
Bis-(3'-5')-cyclic dimeric GMP (c-di-GMP) is an intracellular second messenger that controls the lifestyles of many bacteria. A high intracellular level of c-di-GMP induces a biofilm lifestyle, whereas a low intracellular level of c-di-GMP stimulates dispersal of biofilms and promotes a planktonic lifestyle. Here, we used the expression of different reporters to show that planktonic cells, biofilm cells, and cells dispersed from biofilms (DCells) had distinct intracellular c-di-GMP levels. Proteomics analysis showed that the low intracellular c-di-GMP level of DCells induced the expression of proteins required for the virulence and development of antimicrobial peptide resistance in Pseudomonas aeruginosa. In accordance with this, P. aeruginosa cells with low c-di-GMP levels were found to be more resistant to colistin than P. aeruginosa cells with high c-di-GMP levels. This finding contradicts the current dogma stating that dispersed cells are inevitably more susceptible to antibiotics than their sessile counterparts.
Figures

Similar articles
-
Heterogeneity in surface sensing suggests a division of labor in Pseudomonas aeruginosa populations.Elife. 2019 Jun 10;8:e45084. doi: 10.7554/eLife.45084. Elife. 2019. PMID: 31180327 Free PMC article.
-
Does the mode of dispersion determine the properties of dispersed Pseudomonas aeruginosa biofilm cells?Int J Antimicrob Agents. 2020 Dec;56(6):106194. doi: 10.1016/j.ijantimicag.2020.106194. Epub 2020 Oct 9. Int J Antimicrob Agents. 2020. PMID: 33039591
-
Elevated levels of the second messenger c-di-GMP contribute to antimicrobial resistance of Pseudomonas aeruginosa.Mol Microbiol. 2014 May;92(3):488-506. doi: 10.1111/mmi.12587. Epub 2014 Apr 9. Mol Microbiol. 2014. PMID: 24655293 Free PMC article.
-
Biofilms and Cyclic di-GMP (c-di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and Other Bacteria.J Biol Chem. 2016 Jun 10;291(24):12547-12555. doi: 10.1074/jbc.R115.711507. Epub 2016 Apr 21. J Biol Chem. 2016. PMID: 27129226 Free PMC article. Review.
-
The bacterial second messenger c-di-GMP: probing interactions with protein and RNA binding partners using cyclic dinucleotide analogs.Org Biomol Chem. 2012 Dec 14;10(46):9113-29. doi: 10.1039/c2ob26724a. Epub 2012 Oct 29. Org Biomol Chem. 2012. PMID: 23108253 Free PMC article. Review.
Cited by
-
Elevated c-di-GMP Levels and Expression of the Type III Secretion System Promote Corneal Infection by Pseudomonas aeruginosa.Infect Immun. 2022 Aug 18;90(8):e0006122. doi: 10.1128/iai.00061-22. Epub 2022 Aug 1. Infect Immun. 2022. PMID: 35913171 Free PMC article.
-
Pleiotropic Effects of c-di-GMP Content in Pseudomonas syringae.Appl Environ Microbiol. 2019 May 2;85(10):e00152-19. doi: 10.1128/AEM.00152-19. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30850427 Free PMC article.
-
Imaging Flow Cytometry to Study Biofilm-Associated Microbial Aggregates.Molecules. 2021 Nov 24;26(23):7096. doi: 10.3390/molecules26237096. Molecules. 2021. PMID: 34885675 Free PMC article.
-
Terpenoids from Platostoma rotundifolium (Briq.) A. J. Paton Alter the Expression of Quorum Sensing-Related Virulence Factors and the Formation of Biofilm in Pseudomonas aeruginosa PAO1.Int J Mol Sci. 2017 Jun 14;18(6):1270. doi: 10.3390/ijms18061270. Int J Mol Sci. 2017. PMID: 28613253 Free PMC article.
-
Proteomics As a Tool for Studying Bacterial Virulence and Antimicrobial Resistance.Front Microbiol. 2016 Mar 31;7:410. doi: 10.3389/fmicb.2016.00410. eCollection 2016. Front Microbiol. 2016. PMID: 27065974 Free PMC article. Review.
References
-
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 - PubMed
-
- Yang L, Liu Y, Wu H, Song Z, Hoiby N, Molin S, Givskov M. 2012. Combating biofilms. FEMS Immunol. Med. Microbiol. 65:146–157 - PubMed
-
- Hengge R. 2009. Principles of c-di-GMP signaling in bacteria. Nat. Rev. Microbiol. 7:263–273 - PubMed
-
- Romling U, Gomelsky M, Galperin MY. 2005. C-di-GMP: the dawning of a novel bacterial signaling system. Mol. Microbiol. 57:629–639 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

