PKR regulates proliferation, differentiation, and survival of murine hematopoietic stem/progenitor cells
- PMID: 23403623
- PMCID: PMC3637012
- DOI: 10.1182/blood-2012-09-456400
PKR regulates proliferation, differentiation, and survival of murine hematopoietic stem/progenitor cells
Abstract
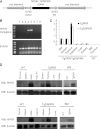
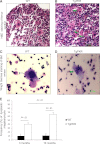
Protein kinase R (PKR) is an interferon (IFN)-inducible, double-stranded RNA-activated kinase that initiates apoptosis in response to cellular stress. To determine the role of PKR in hematopoiesis, we developed transgenic mouse models that express either human PKR (TgPKR) or a dominant-negative PKR (TgDNPKR) mutant specifically in hematopoietic tissues. Significantly, peripheral blood counts from TgPKR mice decrease with age in association with dysplastic marrow changes. TgPKR mice have reduced colony-forming capacity and the colonies also are more sensitive to hematopoietic stresses. Furthermore, TgPKR mice have fewer hematopoietic stem/progenitor cells (HSPCs), and the percentage of quiescent (G0) HSPCs is increased. Importantly, treatment of TgPKR bone marrow (BM) with a PKR inhibitor specifically rescues sensitivity to growth factor deprivation. In contrast, marrow from PKR knockout (PKRKO) mice has increased potential for colony formation and HSPCs are more actively proliferating and resistant to stress. Significantly, TgPKR HSPCs have increased expression of p21 and IFN regulatory factor, whereas cells from PKRKO mice display mechanisms indicative of proliferation such as reduced eukaryotic initiation factor 2α phosphorylation, increased extracellular signal-regulated protein kinases 1 and 2 phosphorylation, and increased CDK2 expression. Collectively, data reveal that PKR is an unrecognized but important regulator of HSPC cell fate and may play a role in the pathogenesis of BM failure.
Figures






References
-
- Blalock WL, Weinstein-Oppenheimer C, Chang F, et al. Signal transduction, cell cycle regulatory, and anti-apoptotic pathways regulated by IL-3 in hematopoietic cells: possible sites for intervention with anti-neoplastic drugs. Leukemia. 1999;13(8):1109–1166. - PubMed
-
- Hapel AJ, Fung MC, Johnson RM, et al. Biologic properties of molecularly cloned and expressed murine interleukin-3. Blood. 1985;65(6):1453–1459. - PubMed
-
- Matsumoto A, Nakayama KI. Role of key regulators of the cell cycle in maintenance of hematopoietic stem cells. Biochim Biophys Acta. 2013;1830(2):2335–2344. - PubMed
-
- Liesveld JL, Jordan CT, Phillips GL., II The hematopoietic stem cell in myelodysplasia. Stem Cells. 2004;22(4):590–599. - PubMed
-
- Lécuyer E, Hoang T. SCL: from the origin of hematopoiesis to stem cells and leukemia. Exp Hematol. 2004;32(1):11–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

