Inhibition of BET bromodomain targets genetically diverse glioblastoma
- PMID: 23403638
- PMCID: PMC4172367
- DOI: 10.1158/1078-0432.CCR-12-3066
Inhibition of BET bromodomain targets genetically diverse glioblastoma
Abstract
Purpose: Glioblastoma is refractory to conventional therapies. The bromodomain and extraterminal domain (BET) proteins are epigenetic readers that selectively bind to acetylated lysine residues on histone tails. These proteins recently emerged as important therapeutic targets in NUT midline carcinoma and several types of hematopoietic cancers. In this study, the therapeutic potential of a novel BET bromodomain inhibitor, JQ1, was assessed in a panel of genetically heterogeneous glioblastoma samples.
Experimental design: The antineoplastic effects of JQ1 were shown using ex vivo cultures derived from primary glioblastoma xenograft lines and surgical specimens of different genetic background. The in vivo efficacy was assessed in orthotopic glioblastoma tumors.
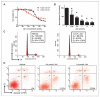
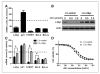
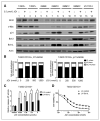
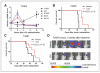
Results: We showed that JQ1 induced marked G1 cell-cycle arrest and apoptosis, which was phenocopied by knockdown of individual BET family members. JQ1 treatment resulted in significant changes in expression of genes that play important roles in glioblastoma such as c-Myc, p21(CIP1/WAF1), hTERT, Bcl-2, and Bcl-xL. Unlike the observations in some hematopoietic cancer cell lines, exogenous c-Myc did not significantly protect glioblastoma cells against JQ1. In contrast, ectopically expressed Bcl-xL partially rescued cells from JQ1-induced apoptosis, and knockdown of p21(CIP1/WAF1) attenuated JQ1-induced cell-cycle arrest. Cells genetically engineered for Akt hyperactivation or p53/Rb inactivation did not compromise JQ1 efficacy, suggesting that these frequently mutated signaling pathways may not confer resistance to JQ1. Furthermore, JQ1 significantly repressed growth of orthotopic glioblastoma tumors.
Conclusion: Our results suggest potentially broad therapeutic use of BET bromodomain inhibitors for treating genetically diverse glioblastoma tumors.
©2013 AACR.
Figures


References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Sathornsumetee S, Rich JN. Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci. 2008;1142:108–32. - PubMed
-
- Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. N Engl J Med. 2012;367:647–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

