Oxidative stress and HPV carcinogenesis
- PMID: 23403708
- PMCID: PMC3640522
- DOI: 10.3390/v5020708
Oxidative stress and HPV carcinogenesis
Abstract
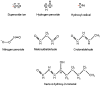
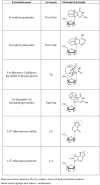
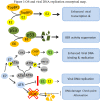
Extensive experimental work has conclusively demonstrated that infection with certain types of human papillomaviruses, the so-called high-risk human papillomavirus (HR-HPV), represent a most powerful human carcinogen. However, neoplastic growth is a rare and inappropriate outcome in the natural history of HPV, and a number of other events have to concur in order to induce the viral infection into the (very rare) neoplastic transformation. From this perspective, a number of putative viral, host, and environmental co-factors have been proposed as potential candidates. Among them oxidative stress (OS) is an interesting candidate, yet comparatively underexplored. OS is a constant threat to aerobic organisms being generated during mitochondrial oxidative phosphorylation, as well as during inflammation, infections, ionizing irradiation, UV exposure, mechanical and chemical stresses. Epithelial tissues, the elective target for HPV infection, are heavily exposed to all named sources of OS. Two different types of cooperative mechanisms are presumed to occur between OS and HPV: I) The OS genotoxic activity and the HPV-induced genomic instability concur independently to the generation of the molecular damage necessary for the emergence of neoplastic clones. This first mode is merely a particular form of co-carcinogenesis; and II) OS specifically interacts with one or more molecular stages of neoplastic initiation and/or progression induced by the HPV infection. This manuscript was designed to summarize available data on this latter hypothesis. Experimental data and indirect evidences on promoting the activity of OS in viral infection and viral integration will be reviewed. The anti-apoptotic and pro-angiogenetic role of NO (nitric oxide) and iNOS (inducible nitric oxide synthase) will be discussed together with the OS/HPV cooperation in inducing cancer metabolism adaptation. Unexplored/underexplored aspects of the OS interplay with the HPV-driven carcinogenesis will be highlighted. The aim of this paper is to stimulate new areas of study and innovative approaches.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

