Beyond the frontiers of neuronal types
- PMID: 23403725
- PMCID: PMC3566547
- DOI: 10.3389/fncir.2013.00013
Beyond the frontiers of neuronal types
Abstract
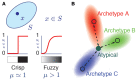
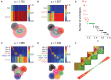
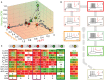
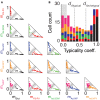
Cortical neurons and, particularly, inhibitory interneurons display a large diversity of morphological, synaptic, electrophysiological, and molecular properties, as well as diverse embryonic origins. Various authors have proposed alternative classification schemes that rely on the concomitant observation of several multimodal features. However, a broad variability is generally observed even among cells that are grouped into a same class. Furthermore, the attribution of specific neurons to a single defined class is often difficult, because individual properties vary in a highly graded fashion, suggestive of continua of features between types. Going beyond the description of representative traits of distinct classes, we focus here on the analysis of atypical cells. We introduce a novel paradigm for neuronal type classification, assuming explicitly the existence of a structured continuum of diversity. Our approach, grounded on the theory of fuzzy sets, identifies a small optimal number of model archetypes. At the same time, it quantifies the degree of similarity between these archetypes and each considered neuron. This allows highlighting archetypal cells, which bear a clear similarity to a single model archetype, and edge cells, which manifest a convergence of traits from multiple archetypes.
Keywords: atypical cells; barrel cortex; fuzzy sets; interneuron diversity; neuronal diversity; petilla terminology; unsupervised clustering.
Figures



Similar articles
-
Single-Cell Stimulation in Barrel Cortex Influences Psychophysical Detection Performance.J Neurosci. 2018 Feb 21;38(8):2057-2068. doi: 10.1523/JNEUROSCI.2155-17.2018. Epub 2018 Jan 22. J Neurosci. 2018. PMID: 29358364 Free PMC article.
-
Inhibitory interneurons and their circuit motifs in the many layers of the barrel cortex.Neuroscience. 2018 Jan 1;368:132-151. doi: 10.1016/j.neuroscience.2017.05.027. Epub 2017 May 18. Neuroscience. 2018. PMID: 28528964 Review.
-
Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex.J Neurosci. 2008 Aug 13;28(33):8273-84. doi: 10.1523/JNEUROSCI.5701-07.2008. J Neurosci. 2008. PMID: 18701690 Free PMC article.
-
Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex.J Neurophysiol. 2006 Aug;96(2):834-45. doi: 10.1152/jn.01079.2005. Epub 2006 May 17. J Neurophysiol. 2006. PMID: 16707715
-
Spike timing and synaptic dynamics at the awake thalamocortical synapse.Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
Cited by
-
Morphological and Functional Characterization of Non-fast-Spiking GABAergic Interneurons in Layer 4 Microcircuitry of Rat Barrel Cortex.Cereb Cortex. 2018 Apr 1;28(4):1439-1457. doi: 10.1093/cercor/bhx352. Cereb Cortex. 2018. PMID: 29329401 Free PMC article.
-
Juxtacellular recording and morphological identification of single neurons in freely moving rats.Nat Protoc. 2014 Oct;9(10):2369-81. doi: 10.1038/nprot.2014.161. Epub 2014 Sep 11. Nat Protoc. 2014. PMID: 25211514
-
Motor directional tuning across brain areas: directional resonance and the role of inhibition for directional accuracy.Front Neural Circuits. 2013 May 15;7:92. doi: 10.3389/fncir.2013.00092. eCollection 2013. Front Neural Circuits. 2013. PMID: 23720612 Free PMC article. Review.
-
Excitatory cholecystokinin neurons of the midbrain integrate diverse temporal responses and drive auditory thalamic subdomains.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2007724118. doi: 10.1073/pnas.2007724118. Proc Natl Acad Sci U S A. 2021. PMID: 33658359 Free PMC article.
-
Synaptic Plasticity in Cortical Inhibitory Neurons: What Mechanisms May Help to Balance Synaptic Weight Changes?Front Cell Neurosci. 2020 Sep 4;14:204. doi: 10.3389/fncel.2020.00204. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33100968 Free PMC article. Review.
References
-
- Basu S., Banerjee A., Mooney R. J. (2002). Semi-supervised clustering by seeding, in Proceedings of the Nineteenth International Conference on Machine Learning, eds Sammut C., Hoffmann A. G. (San Francisco, CA: Morgan Kaufmann; ), 27–34
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

