Design and physicochemical characterization of advanced spray-dried tacrolimus multifunctional particles for inhalation
- PMID: 23403805
- PMCID: PMC3569053
- DOI: 10.2147/DDDT.S40166
Design and physicochemical characterization of advanced spray-dried tacrolimus multifunctional particles for inhalation
Abstract
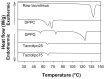
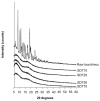
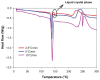
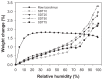
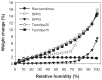
The aim of this study was to design, develop, and optimize respirable tacrolimus microparticles and nanoparticles and multifunctional tacrolimus lung surfactant mimic particles for targeted dry powder inhalation delivery as a pulmonary nanomedicine. Particles were rationally designed and produced at different pump rates by advanced spray-drying particle engineering design from organic solution in closed mode. In addition, multifunctional tacrolimus lung surfactant mimic dry powder particles were prepared by co-dissolving tacrolimus and lung surfactant mimic phospholipids in methanol, followed by advanced co-spray-drying particle engineering design technology in closed mode. The lung surfactant mimic phospholipids were 1,2-dipalmitoyl-sn-glycero-3-phosphocholine and 1,2-dipalmitoyl-sn-glycero-3-[phosphor-rac-1-glycerol]. Laser diffraction particle sizing indicated that the particle size distributions were suitable for pulmonary delivery, whereas scanning electron microscopy imaging indicated that these particles had both optimal particle morphology and surface morphology. Increasing the pump rate percent of tacrolimus solution resulted in a larger particle size. X-ray powder diffraction patterns and differential scanning calorimetry thermograms indicated that spray drying produced particles with higher amounts of amorphous phase. X-ray powder diffraction and differential scanning calorimetry also confirmed the preservation of the phospholipid bilayer structure in the solid state for all engineered respirable particles. Furthermore, it was observed in hot-stage micrographs that raw tacrolimus displayed a liquid crystal transition following the main phase transition, which is consistent with its interfacial properties. Water vapor uptake and lyotropic phase transitions in the solid state at varying levels of relative humidity were determined by gravimetric vapor sorption technique. Water content in the various powders was very low and well within the levels necessary for dry powder inhalation, as quantified by Karl Fisher coulometric titration. Conclusively, advanced spray-drying particle engineering design from organic solution in closed mode was successfully used to design and optimize solid-state particles in the respirable size range necessary for targeted pulmonary delivery, particularly for the deep lung. These particles were dry, stable, and had optimal properties for dry powder inhalation as a novel pulmonary nanomedicine.
Keywords: dry powder inhaler (DPI); immunosuppression; lung surfactant; lung transplant; organic solution advanced spray drying; phospholipid colloidal self-assemblies; pulmonary nanomedicine; solid-state particle engineering design.
Figures





Similar articles
-
Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried cyclosporine A multifunctional particles for dry powder inhalation aerosol delivery.Int J Nanomedicine. 2013;8:1269-83. doi: 10.2147/IJN.S40904. Epub 2013 Mar 24. Int J Nanomedicine. 2013. PMID: 23569375 Free PMC article.
-
Design, physicochemical characterization, and optimization of organic solution advanced spray-dried inhalable dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylethanolamine poly(ethylene glycol) (DPPE-PEG) microparticles and nanoparticles for targeted respiratory nanomedicine delivery as dry powder inhalation aerosols.Int J Nanomedicine. 2013;8:275-93. doi: 10.2147/IJN.S30724. Epub 2013 Jan 15. Int J Nanomedicine. 2013. PMID: 23355776 Free PMC article.
-
Physicochemical characterization and water vapor sorption of organic solution advanced spray-dried inhalable trehalose microparticles and nanoparticles for targeted dry powder pulmonary inhalation delivery.AAPS PharmSciTech. 2011 Dec;12(4):1420-30. doi: 10.1208/s12249-011-9704-0. Epub 2011 Oct 25. AAPS PharmSciTech. 2011. PMID: 22038473 Free PMC article.
-
Recent advances in dry powder inhalation formulations prepared by co-spray drying technology: a comprehensive review.Int J Pharm. 2025 Aug 20;681:125825. doi: 10.1016/j.ijpharm.2025.125825. Epub 2025 Jun 8. Int J Pharm. 2025. PMID: 40494455 Review.
-
Spray-Dried PulmoSphere™ Formulations for Inhalation Comprising Crystalline Drug Particles.AAPS PharmSciTech. 2019 Feb 7;20(3):103. doi: 10.1208/s12249-018-1280-0. AAPS PharmSciTech. 2019. PMID: 30734187 Review.
Cited by
-
Advanced Spray-Dried Inhalable Microparticles/Nanoparticles of an Innovative Mitophagy Activator for Targeted Lung Delivery: Design, Comprehensive Characterization, Human Lung Cell Culture, and In Vitro Aerosol Dispersion Performance.ACS Pharmacol Transl Sci. 2024 Oct 15;7(11):3540-3558. doi: 10.1021/acsptsci.4c00436. eCollection 2024 Nov 8. ACS Pharmacol Transl Sci. 2024. PMID: 39539257
-
Development of a microparticle-based dry powder inhalation formulation of ciprofloxacin hydrochloride applying the quality by design approach.Drug Des Devel Ther. 2016 Oct 12;10:3331-3343. doi: 10.2147/DDDT.S116443. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27784991 Free PMC article.
-
Design, characterization, and aerosol dispersion performance modeling of advanced spray-dried microparticulate/nanoparticulate mannitol powders for targeted pulmonary delivery as dry powder inhalers.J Aerosol Med Pulm Drug Deliv. 2014 Apr;27(2):81-93. doi: 10.1089/jamp.2013.1078. Epub 2014 Feb 6. J Aerosol Med Pulm Drug Deliv. 2014. PMID: 24502451 Free PMC article.
-
Innovative Dual Combination Cospray-Dried Rock Inhibitor/l-Carnitine Inhalable Dry Powder Aerosols.ACS Bio Med Chem Au. 2024 Oct 28;4(6):300-318. doi: 10.1021/acsbiomedchemau.4c00063. eCollection 2024 Dec 18. ACS Bio Med Chem Au. 2024. PMID: 39712207 Free PMC article.
-
Synthesis and Characterization of Nanocomposite Microparticles (nCmP) for the Treatment of Cystic Fibrosis-Related Infections.Pharm Res. 2016 Aug;33(8):1862-72. doi: 10.1007/s11095-016-1921-5. Epub 2016 Apr 18. Pharm Res. 2016. PMID: 27091030 Free PMC article.
References
-
- Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26(8):782–795. - PubMed
-
- Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report-2007. J Heart Lung Transplant. 2007;26(8):769–781. - PubMed
-
- Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22(6):1007–1018. - PubMed
-
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

