Comparative effect sizes in randomised trials from less developed and more developed countries: meta-epidemiological assessment
- PMID: 23403829
- PMCID: PMC3570069
- DOI: 10.1136/bmj.f707
Comparative effect sizes in randomised trials from less developed and more developed countries: meta-epidemiological assessment
Abstract
Objective: To compare treatment effects from randomised trials conducted in more developed versus less developed countries.
Design: Meta-epidemiological study.
Data sources: Cochrane Database of Systematic Reviews (August 2012).
Data extraction: Meta-analyses with mortality outcomes including data from at least one randomised trial conducted in a less developed country and one in a more developed country. Relative risk estimates of more versus less developed countries were compared by calculating the relative relative risks for each topic and the summary relative relative risks across all topics. Similar analyses were performed for the primary binary outcome of each topic.
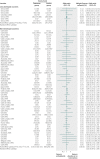
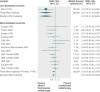
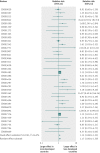
Results: 139 meta-analyses with mortality outcomes were eligible. No nominally significant differences between more developed and less developed countries were found for 128 (92%) meta-analyses. However, differences were beyond chance in 11 (8%) cases, always showing more favourable treatment effects in trials from less developed countries. The summary relative relative risk was 1.12 (95% confidence interval 1.06 to 1.18; P<0.001; I(2)=0%), suggesting significantly more favourable mortality effects in trials from less developed countries. Results were similar for meta-analyses with nominally significant treatment effects for mortality (1.15), meta-analyses with recent trials (1.14), and when excluding trials from less developed countries that subsequently became more developed (1.12). For the primary binary outcomes (127 meta-analyses), 20 topics had differences in treatment effects beyond chance (more favourable in less developed countries in 15/20 cases).
Conclusions: Trials from less developed countries in a few cases show significantly more favourable treatment effects than trials in more developed countries and, on average, treatment effects are more favourable in less developed countries. These discrepancies may reflect biases in reporting or study design as well as genuine differences in baseline risk or treatment implementation and should be considers when generalising evidence across different settings.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures









Similar articles
-
Comparative rates of harms in randomized trials from more developed versus less developed countries may be different.J Clin Epidemiol. 2016 Oct;78:10-21. doi: 10.1016/j.jclinepi.2016.02.032. Epub 2016 Apr 6. J Clin Epidemiol. 2016. PMID: 27063207
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions.Cochrane Database Syst Rev. 2014 Oct 1;2014(10):MR000035. doi: 10.1002/14651858.MR000035.pub2. Cochrane Database Syst Rev. 2014. PMID: 25271098 Free PMC article.
-
Comparative evidence on harms in pediatric randomized clinical trials from less developed versus more developed countries is limited.J Clin Epidemiol. 2018 Mar;95:63-72. doi: 10.1016/j.jclinepi.2017.11.016. Epub 2017 Nov 28. J Clin Epidemiol. 2018. PMID: 29191447 Review.
-
Strategies for optimising antenatal corticosteroid administration for women with anticipated preterm birth.Cochrane Database Syst Rev. 2020 May 26;5(5):CD013633. doi: 10.1002/14651858.CD013633. Cochrane Database Syst Rev. 2020. PMID: 32452555 Free PMC article.
Cited by
-
Assessing risk of bias in randomised clinical trials included in Cochrane Reviews: the why is easy, the how is a challenge.Cochrane Database Syst Rev. 2013 Apr 30;2013(4):ED000058. doi: 10.1002/14651858.ED000058. Cochrane Database Syst Rev. 2013. PMID: 23728703 Free PMC article. No abstract available.
-
Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews.Syst Rev. 2016 May 10;5:80. doi: 10.1186/s13643-016-0259-8. Syst Rev. 2016. PMID: 27160280 Free PMC article. Review.
-
Vitamin D and SARS-CoV-2 virus/COVID-19 disease.BMJ Nutr Prev Health. 2020 May 13;3(1):106-110. doi: 10.1136/bmjnph-2020-000089. eCollection 2020. BMJ Nutr Prev Health. 2020. PMID: 33230499 Free PMC article. No abstract available.
-
Do systematic reviews on pediatric topics need special methodological considerations?BMC Pediatr. 2017 Mar 6;17(1):57. doi: 10.1186/s12887-017-0812-1. BMC Pediatr. 2017. PMID: 28260530 Free PMC article.
-
Language bias in orthodontic systematic reviews: A meta-epidemiological study.PLoS One. 2024 Apr 1;19(4):e0300881. doi: 10.1371/journal.pone.0300881. eCollection 2024. PLoS One. 2024. PMID: 38557691 Free PMC article.
References
-
- Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard School of Public Health, Harvard University Press on behalf of the World Health Organization and The World Bank, 1996.
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291:2720-6. - PubMed
-
- Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol 2007;17:643-53. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
