Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels
- PMID: 23403925
- PMCID: PMC3590989
- DOI: 10.1038/emboj.2013.17
Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels
Abstract
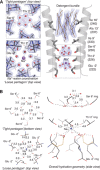
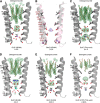
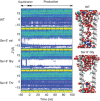
To understand the molecular mechanism of ion permeation in pentameric ligand-gated ion channels (pLGIC), we solved the structure of an open form of GLIC, a prokaryotic pLGIC, at 2.4 Å. Anomalous diffraction data were used to place bound anions and cations. This reveals ordered water molecules at the level of two rings of hydroxylated residues (named Ser6' and Thr2') that contribute to the ion selectivity filter. Two water pentagons are observed, a self-stabilized ice-like water pentagon and a second wider water pentagon, with one sodium ion between them. Single-channel electrophysiology shows that the side-chain hydroxyl of Ser6' is crucial for ion translocation. Simulations and electrostatics calculations complemented the description of hydration in the pore and suggest that the water pentagons observed in the crystal are important for the ion to cross hydrophobic constriction barriers. Simulations that pull a cation through the pore reveal that residue Ser6' actively contributes to ion translocation by reorienting its side chain when the ion is going through the pore. Generalization of these findings to the pLGIC family is proposed.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Abascal JL, Sanz E, Garcia Fernandez R, Vega C (2005) A potential model for the study of ices and amorphous water: TIP4P/Ice. J Chem Phys 122: 234511. - PubMed
-
- Amiri S, Tai K, Beckstein O, Biggin PC, Sansom MS (2005) The alpha7 nicotinic acetylcholine receptor: molecular modelling, electrostatics, and energetics. Mol Membr Biol 22: 151–162 - PubMed
-
- Beckstein O, Sansom MS (2006) A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor. Phys Biol 3: 147–159 - PubMed
-
- Blanc E, Roversi P, Vonrhein C, Flensburg C, Lea SM, Bricogne G (2004) Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr D Biol Crystallogr 60: 2210–2221 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

