pRb is an obesity suppressor in hypothalamus and high-fat diet inhibits pRb in this location
- PMID: 23403926
- PMCID: PMC3604720
- DOI: 10.1038/emboj.2013.25
pRb is an obesity suppressor in hypothalamus and high-fat diet inhibits pRb in this location
Abstract
pRb is frequently inactivated in tumours by mutations or phosphorylation. Here, we investigated whether pRb plays a role in obesity. The Arcuate nucleus (ARC) in hypothalamus contains antagonizing POMC and AGRP/NPY neurons for negative and positive energy balance, respectively. Various aspects of ARC neurons are affected in high-fat diet (HFD)-induced obesity mouse model. Using this model, we show that HFD, as well as pharmacological activation of AMPK, induces pRb phosphorylation and E2F target gene de-repression in ARC neurons. Some affected neurons express POMC; and deleting Rb1 in POMC neurons induces E2F target gene de-repression, cell-cycle re-entry, apoptosis, and a hyperphagia-obesity-diabetes syndrome. These defects can be corrected by combined deletion of E2f1. In contrast, deleting Rb1 in the antagonizing AGRP/NPY neurons shows no effects. Thus, pRb-E2F1 is an obesity suppression mechanism in ARC POMC neurons and HFD-AMPK inhibits this mechanism by phosphorylating pRb in this location.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
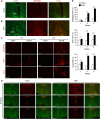

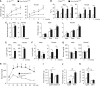



Similar articles
-
Unveiling the transcriptome alteration of POMC neuron in diet-induced obesity.Exp Cell Res. 2020 Apr 1;389(1):111848. doi: 10.1016/j.yexcr.2020.111848. Epub 2020 Jan 16. Exp Cell Res. 2020. PMID: 31954693
-
PNOCARC Neurons Promote Hyperphagia and Obesity upon High-Fat-Diet Feeding.Neuron. 2020 Jun 17;106(6):1009-1025.e10. doi: 10.1016/j.neuron.2020.03.022. Epub 2020 Apr 16. Neuron. 2020. PMID: 32302532 Free PMC article.
-
Cyclin-dependent kinase 4/6 inhibitors require an arcuate-to-paraventricular hypothalamus melanocortin circuit to treat diet-induced obesity.Am J Physiol Endocrinol Metab. 2021 Mar 1;320(3):E467-E474. doi: 10.1152/ajpendo.00386.2020. Epub 2020 Dec 28. Am J Physiol Endocrinol Metab. 2021. PMID: 33356996 Free PMC article.
-
[Advances in the correlation between loss of neural homeostasis and diet-induced obesity].Sheng Wu Gong Cheng Xue Bao. 2019 Aug 25;35(8):1433-1440. doi: 10.13345/j.cjb.190015. Sheng Wu Gong Cheng Xue Bao. 2019. PMID: 31441614 Review. Chinese.
-
Network of hypothalamic neurons that control appetite.BMB Rep. 2015 Apr;48(4):229-33. doi: 10.5483/bmbrep.2015.48.4.272. BMB Rep. 2015. PMID: 25560696 Free PMC article. Review.
Cited by
-
Neuronal Cell Cycle Events Link Caloric Intake to Obesity.Trends Endocrinol Metab. 2020 Jan;31(1):46-52. doi: 10.1016/j.tem.2019.09.001. Epub 2019 Oct 16. Trends Endocrinol Metab. 2020. PMID: 31629614 Free PMC article. Review.
-
Prohibitions in the meta-inflammatory response: a review.Front Mol Biosci. 2024 May 15;11:1322687. doi: 10.3389/fmolb.2024.1322687. eCollection 2024. Front Mol Biosci. 2024. PMID: 38813101 Free PMC article. Review.
-
Exploring the Genetic Conception of Obesity via the Dual Role of FoxO.Int J Mol Sci. 2021 Mar 20;22(6):3179. doi: 10.3390/ijms22063179. Int J Mol Sci. 2021. PMID: 33804729 Free PMC article. Review.
-
Maternal supplementation with konjac glucomannan and κ-carrageenan promotes sow performance and benefits the gut barrier in offspring.Anim Nutr. 2024 Sep 5;19:272-286. doi: 10.1016/j.aninu.2024.05.011. eCollection 2024 Dec. Anim Nutr. 2024. PMID: 39640556 Free PMC article.
-
Skp2 suppresses apoptosis in Rb1-deficient tumours by limiting E2F1 activity.Nat Commun. 2014 Mar 17;5:3463. doi: 10.1038/ncomms4463. Nat Commun. 2014. PMID: 24632684 Free PMC article.
References
-
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR (2008) Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab 7: 377–388 - PubMed
-
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ (2004) AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279: 12005–12008 - PubMed
-
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, Lowell BB (2004) Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42: 983–991 - PubMed
-
- Batsche E, Desroches J, Bilodeau S, Gauthier Y, Drouin J (2005a) Rb enhances p160/SRC coactivator-dependent activity of nuclear receptors and hormone responsiveness. J Biol Chem 280: 19746–19756 - PubMed
-
- Batsche E, Moschopoulos P, Desroches J, Bilodeau S, Drouin J (2005b) Retinoblastoma and the related pocket protein p107 act as coactivators of NeuroD1 to enhance gene transcription. J Biol Chem 280: 16088–16095 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK080003/DK/NIDDK NIH HHS/United States
- 5P30CA13330/CA/NCI NIH HHS/United States
- R01CA87566/CA/NCI NIH HHS/United States
- R01DK057621/DK/NIDDK NIH HHS/United States
- R01 DK061153/DK/NIDDK NIH HHS/United States
- P01 DK052956/DK/NIDDK NIH HHS/United States
- 5P30DK061153/DK/NIDDK NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- R01 DK080003/DK/NIDDK NIH HHS/United States
- R01 CA087566/CA/NCI NIH HHS/United States
- R01 DK057621/DK/NIDDK NIH HHS/United States
- P01DK052956/DK/NIDDK NIH HHS/United States
- P01DK26687/DK/NIDDK NIH HHS/United States
- R01 CA127901/CA/NCI NIH HHS/United States
- P30 DK026687/DK/NIDDK NIH HHS/United States
- R01 DK092246/DK/NIDDK NIH HHS/United States
- R01CA127901/CA/NCI NIH HHS/United States
- R01 CA131421/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

