Effects of 11β-hydroxysteroid dehydrogenase-1 inhibition on hepatic glycogenolysis and gluconeogenesis
- PMID: 23403942
- PMCID: PMC3625750
- DOI: 10.1152/ajpendo.00639.2012
Effects of 11β-hydroxysteroid dehydrogenase-1 inhibition on hepatic glycogenolysis and gluconeogenesis
Abstract
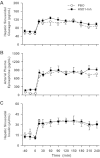
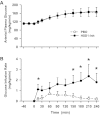
The aim of this study was to determine the effect of prolonged 11β-hydroxysteroid dehydrogenase-1 (11β-HSD1) inhibition on basal and hormone-stimulated glucose metabolism in fasted conscious dogs. For 7 days prior to study, either an 11β-HSD1 inhibitor (HSD1-I; n = 6) or placebo (PBO; n = 6) was administered. After the basal period, a 4-h metabolic challenge followed, where glucagon (3×-basal), epinephrine (5×-basal), and insulin (2×-basal) concentrations were increased. Hepatic glucose fluxes did not differ between groups during the basal period. In response to the metabolic challenge, hepatic glucose production was stimulated in PBO, resulting in hyperglycemia such that exogenous glucose was required in HSD-I (P < 0.05) to match the glycemia between groups. Net hepatic glucose output and endogenous glucose production were decreased by 11β-HSD1 inhibition (P < 0.05) due to a reduction in net hepatic glycogenolysis (P < 0.05), with no effect on gluconeogenic flux compared with PBO. In addition, glucose utilization (P < 0.05) and the suppression of lipolysis were increased (P < 0.05) in HSD-I compared with PBO. These data suggest that inhibition of 11β-HSD1 may be of therapeutic value in the treatment of diseases characterized by insulin resistance and excessive hepatic glucose production.
Figures





Similar articles
-
Effect of 11 beta-hydroxysteroid dehydrogenase-1 inhibition on hepatic glucose metabolism in the conscious dog.Am J Physiol Endocrinol Metab. 2010 May;298(5):E1019-26. doi: 10.1152/ajpendo.00740.2009. Epub 2010 Feb 16. Am J Physiol Endocrinol Metab. 2010. PMID: 20159854 Free PMC article.
-
Inhibition of glycogenolysis enhances gluconeogenic precursor uptake by the liver of conscious dogs.Am J Physiol. 1997 Nov;273(5):E868-79. doi: 10.1152/ajpendo.1997.273.5.E868. Am J Physiol. 1997. PMID: 9374671
-
Direct regulation of glucose and not insulin on hepatic hexose-6-phosphate dehydrogenase and 11β-hydroxysteroid dehydrogenase type 1.Mol Cell Endocrinol. 2011 Feb 10;333(1):62-9. doi: 10.1016/j.mce.2010.12.010. Epub 2010 Dec 14. Mol Cell Endocrinol. 2011. PMID: 21163329 Free PMC article.
-
11beta-hydroxysteroid dehydrogenase type 1 and obesity.Front Horm Res. 2008;36:146-164. doi: 10.1159/000115363. Front Horm Res. 2008. PMID: 18230901 Review.
-
Growth hormone, insulin-like growth factor-I and the cortisol-cortisone shuttle.Horm Res. 2001;56 Suppl 1:1-6. doi: 10.1159/000048126. Horm Res. 2001. PMID: 11786677 Review.
Cited by
-
Tissue-specific dysregulation of cortisol regeneration by 11βHSD1 in obesity: has it promised too much?Diabetologia. 2014 Jun;57(6):1100-10. doi: 10.1007/s00125-014-3228-6. Epub 2014 Apr 8. Diabetologia. 2014. PMID: 24710966 Review.
-
Rictor/mTORC2 facilitates central regulation of energy and glucose homeostasis.Mol Metab. 2014 Feb 19;3(4):394-407. doi: 10.1016/j.molmet.2014.01.014. eCollection 2014 Jul. Mol Metab. 2014. PMID: 24944899 Free PMC article.
-
Hepatic glycogen can regulate hypoglycemic counterregulation via a liver-brain axis.J Clin Invest. 2016 Jun 1;126(6):2236-48. doi: 10.1172/JCI79895. Epub 2016 May 3. J Clin Invest. 2016. PMID: 27140398 Free PMC article.
-
Computational QSAR study of novel 2-aminothiazol-4(5H)-one derivatives as 11β-HSD1 inhibitors.J Comput Aided Mol Des. 2025 Aug 19;39(1):67. doi: 10.1007/s10822-025-00648-7. J Comput Aided Mol Des. 2025. PMID: 40828332 Free PMC article.
-
Effects of Obesity and Insulin on Tissue-Specific Recycling Between Cortisol and Cortisone in Men.J Clin Endocrinol Metab. 2021 Mar 8;106(3):e1206-e1220. doi: 10.1210/clinem/dgaa896. J Clin Endocrinol Metab. 2021. PMID: 33270115 Free PMC article. Clinical Trial.
References
-
- Alberts P, Nilsson C, Selen G, Engblom LO, Edling NH, Norling S, Klingström G, Larsson C, Forsgren M, Ashkzari M, Nilsson CE, Fiedler M, Bergqvist E, Ohman B, Björkstrand E, Abrahmsen LB. Selective inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology 144: 4755–4762, 2003 - PubMed
-
- Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94: 2692–2701, 2009 - PubMed
-
- Andrew R, Smith K, Jones GC, Walker BR. Distinguishing the activities of 11beta-hydroxysteroid dehydrogenases in vivo using isotopically labeled cortisol. J Clin Endocrinol Metab 87: 277–285, 2002 - PubMed
-
- Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 54: 1942–1948, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

