Breast cancer resistance protein regulates apical ectoplasmic specialization dynamics stage specifically in the rat testis
- PMID: 23403943
- PMCID: PMC3625752
- DOI: 10.1152/ajpendo.00645.2012
Breast cancer resistance protein regulates apical ectoplasmic specialization dynamics stage specifically in the rat testis
Abstract
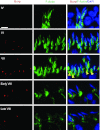
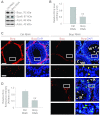
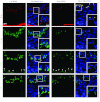
Drug transporters determine the bioavailability of drugs in the testis behind the blood-testis barrier (BTB). Thus, they are crucial for male contraceptive development if these drugs (e.g., adjudin) exert their effects behind the BTB. Herein breast cancer resistance protein (Bcrp), an efflux drug transporter, was found to be expressed by both Sertoli and germ cells. Interestingly, Bcrp was not a component of the Sertoli cell BTB. Instead, it was highly expressed by peritubular myoid cells at the tunica propria and also endothelial cells of the microvessels in the interstitium at all stages of the epithelial cycle. Unexpectedly, Bcrp was found to be expressed at the Sertoli-step 18-19 spermatid interface but limited to stage VI-early VIII tubules, and an integrated component of the apical ectoplasmic specialization (apical ES). Apparently, Bcrp is being used by late-stage spermatids to safeguard their completion of spermiogenesis by preventing harmful drugs to enter these cells while they transform to spermatozoa. Also, the association of Bcrp with actin, Eps8 (epidermal growth factor receptor pathway substrate 8, an actin barbed end capping and bundling protein), and Arp3 (actin-related protein 3, a component of the Arp2/3 complex known to induce branched actin polymerization) at the apical ES suggest that Bcrp may be involved in regulating the organization of actin filament bundles at the site. Indeed, a knockdown of Bcrp by RNAi in the testis perturbed the apical ES function, disrupting spermatid polarity and adhesion. In summary, Bcrp is a regulator of the F-actin-rich apical ES in the testis.
Figures




Similar articles
-
Interaction of oligomeric breast cancer resistant protein (BCRP) with adjudin: a male contraceptive with anti-cancer activity.Curr Mol Pharmacol. 2014;7(2):147-53. doi: 10.2174/1874467208666150126154049. Curr Mol Pharmacol. 2014. PMID: 25620224 Free PMC article. Review.
-
Fascin 1 is an actin filament-bundling protein that regulates ectoplasmic specialization dynamics in the rat testis.Am J Physiol Endocrinol Metab. 2014 Nov 1;307(9):E738-53. doi: 10.1152/ajpendo.00113.2014. Epub 2014 Aug 26. Am J Physiol Endocrinol Metab. 2014. PMID: 25159326 Free PMC article.
-
p-FAK-Tyr(397) regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization.Am J Physiol Endocrinol Metab. 2013 Sep 15;305(6):E687-99. doi: 10.1152/ajpendo.00254.2013. Epub 2013 Jul 23. Am J Physiol Endocrinol Metab. 2013. PMID: 23880313 Free PMC article.
-
c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes.Int J Biochem Cell Biol. 2011 Apr;43(4):651-65. doi: 10.1016/j.biocel.2011.01.008. Epub 2011 Jan 21. Int J Biochem Cell Biol. 2011. PMID: 21256972 Free PMC article.
-
Breast cancer resistance protein (Bcrp) and the testis--an unexpected turn of events.Asian J Androl. 2013 Jul;15(4):455-60. doi: 10.1038/aja.2013.24. Epub 2013 May 13. Asian J Androl. 2013. PMID: 23665760 Free PMC article. Review.
Cited by
-
Adjudin disrupts spermatogenesis by targeting drug transporters: Lesson from the breast cancer resistance protein (BCRP).Spermatogenesis. 2013 Apr 1;3(2):e24993. doi: 10.4161/spmg.24993. Spermatogenesis. 2013. PMID: 23885306 Free PMC article.
-
Interaction of oligomeric breast cancer resistant protein (BCRP) with adjudin: a male contraceptive with anti-cancer activity.Curr Mol Pharmacol. 2014;7(2):147-53. doi: 10.2174/1874467208666150126154049. Curr Mol Pharmacol. 2014. PMID: 25620224 Free PMC article. Review.
-
Drug Transporters at the Human Blood-Testis Barrier.Drug Metab Dispos. 2023 May;51(5):560-571. doi: 10.1124/dmd.122.001186. Epub 2023 Feb 2. Drug Metab Dispos. 2023. PMID: 36732077 Free PMC article. Review.
-
Effective Delivery of Male Contraceptives Behind the Blood-Testis Barrier (BTB) - Lesson from Adjudin.Curr Med Chem. 2016;23(7):701-13. doi: 10.2174/0929867323666160112122724. Curr Med Chem. 2016. PMID: 26758796 Free PMC article. Review.
-
In Vitro and In Vivo Models for Drug Transport Across the Blood-Testis Barrier.Drug Metab Dispos. 2023 Sep;51(9):1157-1168. doi: 10.1124/dmd.123.001288. Epub 2023 May 31. Drug Metab Dispos. 2023. PMID: 37258305 Free PMC article. Review.
References
-
- Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol 21: 350–356, 2010 - PubMed
-
- Aravindan GR, Pineau C, Bardin CW, Cheng CY. Ability of trypsin in mimicking germ cell factors that affect Sertoli cell secretory function. J Cell Physiol 168: 123–133, 1996 - PubMed
-
- Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer 40: 2064–2070, 2004 - PubMed
-
- Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol 763: 70–84, 2012 - PubMed
-
- Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/Fer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod 69: 656–672, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

