Characterization of β-cell plasticity mechanisms induced in mice by a transient source of exogenous insulin
- PMID: 23403947
- PMCID: PMC3625751
- DOI: 10.1152/ajpendo.00304.2012
Characterization of β-cell plasticity mechanisms induced in mice by a transient source of exogenous insulin
Abstract
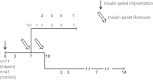
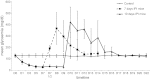
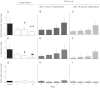
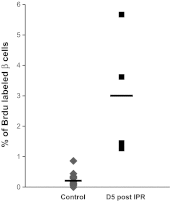
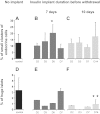
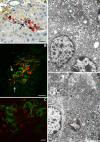
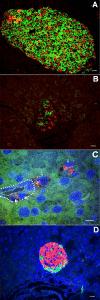
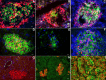
β-Cell plasticity governs the adjustment of β-cell mass and function to ensure normoglycemia. The study of how β-cell mass is controlled and the identification of alternative sources of β-cells are active fields of research. β-Cell plasticity has been implicated in numerous physiological and pathological conditions. We developed a mice model in which we induced major β-cell mass atrophy by implanting insulin pellets (IPI) for 7 or 10 days. The implants were then removed (IPR) to observe the timing and characteristics of β-cell regeneration in parallel to changes in glycemia. Following IPR, the endocrine mass was reduced by 60% at day 7 and by 75% at day 10, and transient hyperglycemia was observed, which resolved within 1 wk. Five days after IPR, enhanced β-cell proliferation and an increased frequency of small islets were observed in 7-day IPI mice. β-Cell mass was fully restored after an additional 2 days. For the 10-day IPI group, β-cell and endocrine mass were no longer significantly different from those of the control group at 2 wk post-IPR. Furthermore, real-time quantitative PCR analysis of endocrine structures isolated by laser capture microdissection indicated sequentially enhanced expression of the pancreatic transcription factors β(2)/NeuroD and Pdx-1 post-IPR. Thus, our data suggest this mouse model of β-cell plasticity not only relies on replication but also involves enhanced cell differentiation plasticity.
Figures

Similar articles
-
Enhanced beta-cell mass without increased proliferation following chronic mild glucose infusion.Am J Physiol Endocrinol Metab. 2008 Apr;294(4):E679-87. doi: 10.1152/ajpendo.00569.2007. Epub 2008 Jan 29. Am J Physiol Endocrinol Metab. 2008. PMID: 18230696
-
Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse.Diabetes. 2006 Dec;55(12):3289-98. doi: 10.2337/db06-0017. Diabetes. 2006. PMID: 17130472
-
The Effects of Empagliflozin, an SGLT2 Inhibitor, on Pancreatic β-Cell Mass and Glucose Homeostasis in Type 1 Diabetes.PLoS One. 2016 Jan 25;11(1):e0147391. doi: 10.1371/journal.pone.0147391. eCollection 2016. PLoS One. 2016. PMID: 26807719 Free PMC article.
-
Pancreatic β-cell regeneration: From molecular mechanisms to therapy.J Cell Biochem. 2019 Sep;120(9):14189-14200. doi: 10.1002/jcb.28834. Epub 2019 May 12. J Cell Biochem. 2019. PMID: 31081169 Review.
-
beta-cell failure in diabetes and preservation by clinical treatment.Endocr Rev. 2007 Apr;28(2):187-218. doi: 10.1210/10.1210/er.2006-0038. Epub 2007 Mar 12. Endocr Rev. 2007. PMID: 17353295 Review.
Cited by
-
Prolonged insulin-induced hypoglycaemia reduces ß-cell activity rather than number in pancreatic islets in non-diabetic rats.Sci Rep. 2022 Aug 18;12(1):14113. doi: 10.1038/s41598-022-18398-z. Sci Rep. 2022. PMID: 35982111 Free PMC article.
References
-
- Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol 24: 297–302, 2000 - PubMed
-
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 38: 49–53, 1989 - PubMed
-
- Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev 85: 1255–1270, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

