Delta-opioid agonist SNC-121 protects retinal ganglion cell function in a chronic ocular hypertensive rat model
- PMID: 23404122
- PMCID: PMC3626520
- DOI: 10.1167/iovs.12-10741
Delta-opioid agonist SNC-121 protects retinal ganglion cell function in a chronic ocular hypertensive rat model
Abstract
Purpose: This study examined if the delta-opioid (δ-opioid) receptor agonist, SNC-121, can improve retinal function and retinal ganglion cell (RGC) survival during glaucomatous injury in a chronic ocular hypertensive rat model.
Methods: IOP was raised in brown Norway rats by injecting hypertonic saline into the limbal venous system. Rats were treated with 1 mg/kg SNC-121 (intraperitoneally [IP]) once daily for 7 days. Pattern-electroretinograms (PERGs) were obtained in response to contrast reversal of patterned visual stimuli. RGCs were visualized by fluorogold retrograde labeling. Expression of TNF-α and p38 mitogen-activated protein (MAP) kinase was measured by immunohistochemistry and Western blotting.
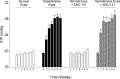
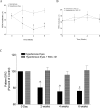
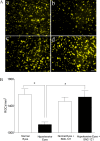
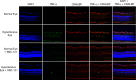
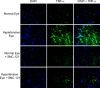
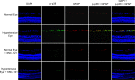
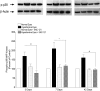
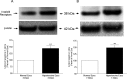
Results: PERG amplitudes in ocular hypertensive eyes were significantly reduced (14.3 ± 0.60 μvolts) when compared with healthy eyes (18.0 ± 0.62 μvolts). PERG loss in hypertensive eyes was inhibited by SNC-121 treatment (17.20 ± 0.1.3 μvolts; P < 0.05). There was a 29% loss of RGCs in the ocular hypertensive eye, which was inhibited in the presence of SNC-121. TNF-α production and activation of p38 MAP kinase in retinal sections and optic nerve samples were upregulated in ocular hypertensive eyes and inhibited in the presence of SNC-121. Furthermore, TNF-α induced increase in p38 MAP kinase activation in astrocytes was inhibited in the presence of SNC-121.
Conclusions: These data provide evidence that activation of δ-opioid receptors inhibited the loss of PERG amplitudes and rate of RGC loss during glaucomatous injury. Mechanistic data provided clues that TNF-α is mainly produced from glial cells and activates p38 MAP kinase, which was significantly inhibited by SNC-121 treatment. Overall, data indicate that enhancement of δ-opioidergic activity in the eye may provide retina neuroprotection against glaucoma.
Conflict of interest statement
Disclosure:
Figures



References
-
- Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979; 86: 1803–1830 - PubMed
-
- Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007; 16: 406–418 - PubMed
-
- Liu Q, Ju WK, Crowston JG, et al. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 2007; 48: 4580–4589 - PubMed
-
- Nucci C, Tartaglione R, Rombola L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005; 26: 935–941 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

