Determination of thalidomide concentration in human plasma by liquid chromatography-tandem mass spectrometry
- PMID: 23404219
- PMCID: PMC3570145
- DOI: 10.3892/etm.2012.847
Determination of thalidomide concentration in human plasma by liquid chromatography-tandem mass spectrometry
Abstract
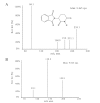
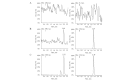
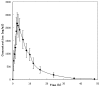
A rapid, sensitive and specific analytical method based on high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) has been developed for the determination of thalidomide concentration in human plasma. The analyte and internal standard were extracted by liquid-liquid extraction with ether-dichloromethane (3:2, v/v) and separated on a TC-C(18) column using methanol-10 mM ammonium acetate-formic acid (60:40:0.04, v/v/v) as the mobile phase at a flow rate of 0.9 ml/min. The detection was performed using an API 4000 triple quadrupole mass spectrometer in the positive electrospray ionization (ESI) mode and completed within 3.0 min. The multiple reaction monitoring (MRM) transitions were m/z 259.1→84.0 for the analyte and m/z 195.9→138.9 for temozolomide. The calibration curve exhibited a linear dynamic range of 2-1500 ng/ml (r>0.9991). The intra-and inter-day precisions (as relative standard deviation; RSD) were 6.8-13.5% and 4.3-5.0% respectively and the accuracy (as relative error; RE) was 2.0-3.5%. The recoveries and matrix effects were satisfactory in all the biological matrices examined. This method was successfully used in a pharmacokinetic study of thalidomide in healthy male volunteers receiving an oral administration of a 200-mg dose.
Keywords: human plasma; liquid chromatography-tandem mass spectrometry; multiple reaction monitoring; thalidomide.
Figures
References
-
- Calabrese L, Resztak K. Thalidomide revisited: pharmacology and clinical applications. Expert Opin Investig Drugs. 1998;7:2043–2060. - PubMed
-
- Laffitte E, Revuz J. Thalidomide: an old drug with new clinical applications. Expert Opin Drug Saf. 2004;3:47–56. - PubMed
-
- Kamikawa R, Ikawa K, Morikawa N, Asaoku H, Iwato K, Sasaki A. The pharmacokinetics of low-dose thalidomide in Japanese patients with refractory multiple myeloma. Biol Pharm Bull. 2006;29:2331–2334. - PubMed
-
- Yakoub-Agha I, Attal M, Dumontet C, et al. Thalidomide in patients with advanced multiple myeloma: a study of 83 patients - report of the Intergroupe Francophone du Myélome (IFM) Hematol J. 2002;3:185–192. - PubMed
-
- Barillé-Nion S, Barlogie B, Bataille R, et al. Advances in biology and therapy of multiple myeloma. Hematology. Am Soc Hematol Educ Program. 2003:248–278. - PubMed
LinkOut - more resources
Full Text Sources
Medical
