Kdo hydrolase is required for Francisella tularensis virulence and evasion of TLR2-mediated innate immunity
- PMID: 23404403
- PMCID: PMC3573668
- DOI: 10.1128/mBio.00638-12
Kdo hydrolase is required for Francisella tularensis virulence and evasion of TLR2-mediated innate immunity
Abstract
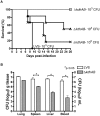
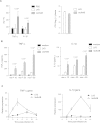
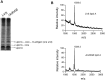
The highly virulent Francisella tularensis subsp. tularensis has been classified as a category A bioterrorism agent. A live vaccine strain (LVS) has been developed but remains unlicensed in the United States because of an incomplete understanding of its attenuation. Lipopolysaccharide (LPS) modification is a common strategy employed by bacterial pathogens to avoid innate immunity. A novel modification enzyme has recently been identified in F. tularensis and Helicobacter pylori. This enzyme, a two-component Kdo (3-deoxy-d-manno-octulosonic acid) hydrolase, catalyzes the removal of a side chain Kdo sugar from LPS precursors. The biological significance of this modification has not yet been studied. To address the role of the two-component Kdo hydrolase KdhAB in F. tularensis pathogenesis, a ΔkdhAB deletion mutant was constructed from the LVS strain. In intranasal infection of mice, the ΔkdhAB mutant strain had a 50% lethal dose (LD(50)) 2 log(10) units higher than that of the parental LVS strain. The levels of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) in bronchoalveolar lavage fluid were significantly higher (2-fold) in mice infected with the ΔkdhAB mutant than in mice infected with LVS. In vitro stimulation of bone marrow-derived macrophages with the ΔkdhAB mutant induced higher levels of TNF-α and IL-1β in a TLR2-dependent manner. In addition, TLR2(-/-) mice were more susceptible than wild-type mice to ΔkdhAB bacterial infection. Finally, immunization of mice with ΔkdhAB bacteria elicited a high level of protection against the highly virulent F. tularensis subsp. tularensis strain Schu S4. These findings suggest an important role for the Francisella Kdo hydrolase system in virulence and offer a novel mutant as a candidate vaccine.
Importance: The first line of defense against a bacterial pathogen is innate immunity, which slows the progress of infection and allows time for adaptive immunity to develop. Some bacterial pathogens, such as Francisella tularensis, suppress the early innate immune response, killing the host before adaptive immunity can mature. To avoid an innate immune response, F. tularensis enzymatically modifies its lipopolysaccharide (LPS). A novel LPS modification-Kdo (3-deoxy-d-manno-octulosonic acid) saccharide removal--has recently been reported in F. tularensis. We found that the kdhAB mutant was significantly attenuated in mice. Additionally, the mutant strain induced an early innate immune response in mice both in vitro and in vivo. Immunization of mice with this mutant provided protection against the highly virulent F. tularensis strain Schu S4. Thus, our study has identified a novel LPS modification important for microbial virulence. A mutant lacking this modification may be used as a live attenuated vaccine against tularemia.
Figures



References
-
- Bosio CM, Bielefeldt-Ohmann H, Belisle JT. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178:4538–4547 - PubMed
-
- Mares CA, Ojeda SS, Morris EG, Li Q, Teale JM. 2008. Initial delay in the immune response to Francisella tularensis is followed by hypercytokinemia characteristic of severe sepsis and correlating with upregulation and release of damage-associated molecular patterns. Infect. Immun. 76:3001–3010 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
