Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of cAMP-response element-binding protein (CREB): implications for Alzheimer disease therapy
- PMID: 23404502
- PMCID: PMC3605648
- DOI: 10.1074/jbc.M112.426536
Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of cAMP-response element-binding protein (CREB): implications for Alzheimer disease therapy
Abstract
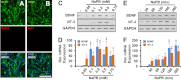
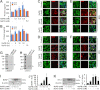
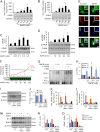
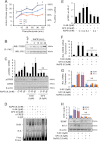
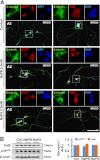
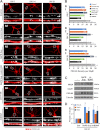
Neurotrophins, such as brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), are believed to be genuine molecular mediators of neuronal growth and homeostatic synapse activity. However, levels of these neurotrophic factors decrease in different brain regions of patients with Alzheimer disease (AD). Induction of astrocytic neurotrophin synthesis is a poorly understood phenomenon but represents a plausible therapeutic target because neuronal neurotrophin production is aberrant in AD and other neurodegenerative diseases. Here, we delineate that sodium phenylbutyrate (NaPB), a Food and Drug Administration-approved oral medication for hyperammonemia, induces astrocytic BDNF and NT-3 expression via the protein kinase C (PKC)-cAMP-response element-binding protein (CREB) pathway. NaPB treatment increased the direct association between PKC and CREB followed by phosphorylation of CREB (Ser(133)) and induction of DNA binding and transcriptional activation of CREB. Up-regulation of markers for synaptic function and plasticity in cultured hippocampal neurons by NaPB-treated astroglial supernatants and its abrogation by anti-TrkB blocking antibody suggest that NaPB-induced astroglial neurotrophins are functionally active. Moreover, oral administration of NaPB increased the levels of BDNF and NT-3 in the CNS and improved spatial learning and memory in a mouse model of AD. Our results highlight a novel neurotrophic property of NaPB that may be used to augment neurotrophins in the CNS and improve synaptic function in disease states such as AD.
Figures



References
-
- Lewin G. R., Barde Y. A. (1996) Physiology of the neurotrophins. Annu. Rev. Neurosci. 19, 289–317 - PubMed
-
- McAllister A. K., Katz L. C., Lo D. C. (1999) Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318 - PubMed
-
- Sofroniew M. V., Howe C. L., Mobley W. C. (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 24, 1217–1281 - PubMed
-
- Chao M. V. (2003) Neurotrophins and their receptors. A convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 - PubMed
-
- Thoenen H. (1995) Neurotrophins and neuronal plasticity. Science 270, 593–598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

