A pathophysiologic role for epidermal growth factor receptor in pemphigus acantholysis
- PMID: 23404504
- PMCID: PMC3611014
- DOI: 10.1074/jbc.M112.438010
A pathophysiologic role for epidermal growth factor receptor in pemphigus acantholysis
Abstract
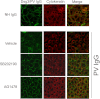
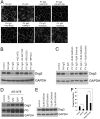
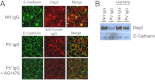
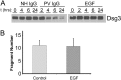
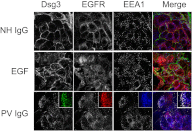
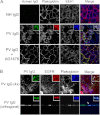
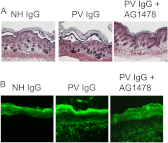
The pemphigus family of autoimmune bullous disorders is characterized by autoantibody binding to desmoglein 1 and/or 3 (dsg1/dsg3). In this study we show that EGF receptor (EGFR) is activated following pemphigus vulgaris (PV) IgG treatment of primary human keratinocytes and that EGFR activation is downstream of p38 mitogen-activated protein kinase (p38). Inhibition of EGFR blocked PV IgG-triggered dsg3 endocytosis, keratin intermediate filament retraction, and loss of cell-cell adhesion in vitro. Significantly, inhibiting EGFR prevented PV IgG-induced blister formation in the passive transfer mouse model of pemphigus. These data demonstrate cross-talk between dsg3 and EGFR, that this cross-talk is regulated by p38, and that EGFR is a potential therapeutic target for pemphigus. Small-molecule inhibitors and monoclonal antibodies directed against EGFR are currently used to treat several types of solid tumors. This study provides the experimental rationale for investigating the use of EGFR inhibitors in pemphigus.
Figures


Similar articles
-
EGFR Inhibition by Erlotinib Rescues Desmosome Ultrastructure and Keratin Anchorage and Protects against Pemphigus Vulgaris IgG-Induced Acantholysis in Human Epidermis.J Invest Dermatol. 2024 Nov;144(11):2440-2452. doi: 10.1016/j.jid.2024.03.040. Epub 2024 Apr 19. J Invest Dermatol. 2024. PMID: 38642796
-
IgG binds to desmoglein 3 in desmosomes and causes a desmosomal split without keratin retraction in a pemphigus mouse model.J Invest Dermatol. 2004 May;122(5):1145-53. doi: 10.1111/j.0022-202X.2004.22426.x. J Invest Dermatol. 2004. PMID: 15140217
-
Keratins Regulate p38MAPK-Dependent Desmoglein Binding Properties in Pemphigus.Front Immunol. 2018 Mar 19;9:528. doi: 10.3389/fimmu.2018.00528. eCollection 2018. Front Immunol. 2018. PMID: 29616033 Free PMC article.
-
New insights into desmosome regulation and pemphigus blistering as a desmosome-remodeling disease.Kaohsiung J Med Sci. 2013 Jan;29(1):1-13. doi: 10.1016/j.kjms.2012.08.001. Epub 2012 Oct 12. Kaohsiung J Med Sci. 2013. PMID: 23257250 Free PMC article. Review.
-
Mechanisms Causing Acantholysis in Pemphigus-Lessons from Human Skin.Front Immunol. 2022 May 20;13:884067. doi: 10.3389/fimmu.2022.884067. eCollection 2022. Front Immunol. 2022. PMID: 35720332 Free PMC article. Review.
Cited by
-
Desmosomes as Signaling Hubs in the Regulation of Cell Behavior.Front Cell Dev Biol. 2021 Sep 23;9:745670. doi: 10.3389/fcell.2021.745670. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34631720 Free PMC article. Review.
-
Interference RNA in immune-mediated oral diseases - minireview.Cent Eur J Immunol. 2017;42(3):301-304. doi: 10.5114/ceji.2017.70974. Epub 2017 Oct 30. Cent Eur J Immunol. 2017. PMID: 29204096 Free PMC article. Review.
-
What protein kinases are crucial for acantholysis and blister formation in pemphigus vulgaris? A systematic review.J Cell Physiol. 2022 Jul;237(7):2825-2837. doi: 10.1002/jcp.30784. Epub 2022 May 26. J Cell Physiol. 2022. PMID: 35616233 Free PMC article.
-
Modulation of Mechanical Stress Mitigates Anti-Dsg3 Antibody-Induced Dissociation of Cell-Cell Adhesion.Adv Biol (Weinh). 2021 Jan;5(1):e2000159. doi: 10.1002/adbi.202000159. Epub 2021 Jan 4. Adv Biol (Weinh). 2021. PMID: 33724731 Free PMC article.
-
Immune response in pemphigus and beyond: progresses and emerging concepts.Semin Immunopathol. 2016 Jan;38(1):57-74. doi: 10.1007/s00281-015-0541-1. Epub 2015 Nov 23. Semin Immunopathol. 2016. PMID: 26597100 Review.
References
-
- Stanley J. R., Koulu L., Klaus-Kovtun V., Steinberg M. S. (1986) A monoclonal antibody to the desmosomal glycoprotein desmoglein I binds the same polypeptide as human autoantibodies in pemphigus foliaceus. J. Immunol. 136, 1227–1230 - PubMed
-
- Amagai M., Klaus-Kovtun V., Stanley J. R. (1991) Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell 67, 869–877 - PubMed
-
- Seishima M., Iwasaki-Bessho Y., Itoh Y., Nozawa Y., Amagai M., Kitajima Y. (1999) Phosphatidylcholine-specific phospholipase C, but not phospholipase D, is involved in pemphigus IgG-induced signal transduction. Arch. Dermatol. Res. 291, 606–613 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

