The propeptides of VEGF-D determine heparin binding, receptor heterodimerization, and effects on tumor biology
- PMID: 23404505
- PMCID: PMC3605636
- DOI: 10.1074/jbc.M112.439299
The propeptides of VEGF-D determine heparin binding, receptor heterodimerization, and effects on tumor biology
Abstract
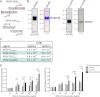
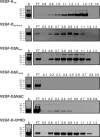
VEGF-D is an angiogenic and lymphangiogenic glycoprotein that can be proteolytically processed generating various forms differing in subunit composition due to the presence or absence of N- and C-terminal propeptides. These propeptides flank the central VEGF homology domain, that contains the binding sites for VEGF receptors (VEGFRs), but their biological functions were unclear. Characterization of propeptide function will be important to clarify which forms of VEGF-D are biologically active and therefore clinically relevant. Here we use VEGF-D mutants deficient in either propeptide, and in the capacity to process the remaining propeptide, to monitor the functions of these domains. We report for the first time that VEGF-D binds heparin, and that the C-terminal propeptide significantly enhances this interaction (removal of this propeptide from full-length VEGF-D completely prevents heparin binding). We also show that removal of either the N- or C-terminal propeptide is required for VEGF-D to drive formation of VEGFR-2/VEGFR-3 heterodimers which have recently been shown to positively regulate angiogenic sprouting. The mature form of VEGF-D, lacking both propeptides, can also promote formation of these receptor heterodimers. In a mouse tumor model, removal of only the C-terminal propeptide from full-length VEGF-D was sufficient to enhance angiogenesis and tumor growth. In contrast, removal of both propeptides is required for high rates of lymph node metastasis. The findings reported here show that the propeptides profoundly influence molecular interactions of VEGF-D with VEGF receptors, co-receptors, and heparin, and its effects on tumor biology.
Figures



Similar articles
-
Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D.J Biol Chem. 2016 Dec 30;291(53):27265-27278. doi: 10.1074/jbc.M116.736801. Epub 2016 Nov 16. J Biol Chem. 2016. PMID: 27852824 Free PMC article.
-
Proteolytic processing of vascular endothelial growth factor-D is essential for its capacity to promote the growth and spread of cancer.FASEB J. 2011 Aug;25(8):2615-25. doi: 10.1096/fj.10-179788. Epub 2011 Apr 22. FASEB J. 2011. PMID: 21515745
-
Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2.FASEB J. 2007 Apr;21(4):1088-98. doi: 10.1096/fj.06-7060com. Epub 2007 Jan 22. FASEB J. 2007. PMID: 17242158
-
[Vascular endothelial growth factor (VEGF)-D in association with VEGF receptor-3 in lymphatic metastasis of breast cancer].Ai Zheng. 2009 Dec;28(12):1337-43. doi: 10.5732/cjc.009.10070. Ai Zheng. 2009. PMID: 19958632 Review. Chinese.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
Cited by
-
Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors.Int J Mol Sci. 2023 Aug 28;24(17):13317. doi: 10.3390/ijms241713317. Int J Mol Sci. 2023. PMID: 37686121 Free PMC article. Review.
-
Sulfatase 2 facilitates lymphangiogenesis in breast cancer by regulating VEGF-D.Oncol Rep. 2016 Dec;36(6):3161-3171. doi: 10.3892/or.2016.5143. Epub 2016 Oct 4. Oncol Rep. 2016. PMID: 27748846 Free PMC article.
-
GSEA-SDBE: A gene selection method for breast cancer classification based on GSEA and analyzing differences in performance metrics.PLoS One. 2022 Apr 26;17(4):e0263171. doi: 10.1371/journal.pone.0263171. eCollection 2022. PLoS One. 2022. PMID: 35472078 Free PMC article.
-
Pulmonary Vasculopathy Associated with FIGF Gene Mutation.Am J Pathol. 2017 Jan;187(1):25-32. doi: 10.1016/j.ajpath.2016.09.008. Epub 2016 Nov 12. Am J Pathol. 2017. PMID: 27846380 Free PMC article.
-
Sulfatase 2 promotes breast cancer progression through regulating some tumor-related factors.Oncol Rep. 2016 Mar;35(3):1318-28. doi: 10.3892/or.2015.4525. Epub 2015 Dec 28. Oncol Rep. 2016. PMID: 26708018 Free PMC article.
References
-
- Tammela T., Alitalo K. (2010) Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140, 460–476 - PubMed
-
- Achen M. G., McColl B. K., Stacker S. A. (2005) Focus on lymphangiogenesis in tumor metastasis. Cancer Cell 7, 121–127 - PubMed
-
- Potente M., Gerhardt H., Carmeliet P. (2011) Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 - PubMed
-
- Seyama K., Kumasaka T., Kurihara M., Mitani K., Sato T. (2010) Lymphangioleiomyomatosis: a disease involving the lymphatic system. Lymphat. Res. Biol. 8, 21–31 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

