The Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE) study: 2-year nonvertebral fragility fracture results
- PMID: 23404615
- PMCID: PMC3706736
- DOI: 10.1007/s00198-013-2284-y
The Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE) study: 2-year nonvertebral fragility fracture results
Abstract
This observational study evaluated the occurrence of nonvertebral fragility fractures (NVFX) in over 4,000 men and women with osteoporosis treated with teriparatide (TPTD). The incidence of new NVFX decreased for patients receiving TPTD treatment for greater than 6 months. No new significant safety findings were observed in this large trial.
Introduction: The Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE) study evaluated the occurrence of NVFX in patients receiving TPTD for osteoporosis in a real-world setting.
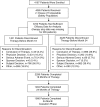
Methods: DANCE is a multicenter, prospective, observational trial that examined the long-term effectiveness of TPTD in men and women with osteoporosis whom study physicians judged to be suitable for TPTD therapy. Patients received 20 μg TPTD per day by subcutaneous injection for up to 24 months and were followed for 24 months after treatment cessation. The incidence of patients experiencing a new NVFX, defined as a fracture associated with low trauma, was evaluated during four 6-month periods in both the treatment and cessation phases with >0 to ≤6 months serving as the reference. We also observed the spectrum and occurrence of serious adverse events.
Results: Of the 4,167 patients enrolled, 4,085 took one or more doses of TPTD (safety population); 3,720 were included in the efficacy analysis. The incidence of patients experiencing a NVFX was 1.42, 0.91, 0.70, and 0.81 % for the four treatment periods, respectively, and 0.80, 0.68, 0.33, and 0.33 % for the four periods after treatment cessation. Differences for each period were statistically significant compared with the reference period (first 6-month interval, each p < 0.05). No new significant safety findings were observed.
Conclusions: In this study, the incidence of NVFX decreased for patients receiving TPTD for all three treatment periods >6 months compared to 0 to ≤6 months, and this trend persisted throughout the cessation phase. TPTD was generally well tolerated.
Figures
References
-
- Langdahl BL, Rajzbaum G, Jakob F, Karras D, Ljunggren O, Lems WF, Fahrleitner-Pammer A, Walsh JB, Barker C, Kutahov A, Marin F. Reduction in fracture rate and back pain and increased quality of life in postmenopausal women treated with teriparatide: 18-month data from the European Forsteo Observational Study (EFOS) Calcif Tissue Int. 2009;85:484–493. doi: 10.1007/s00223-009-9299-6. - DOI - PMC - PubMed
-
- National Institutes of Health (2011) NIH website: http://www.ncbi.nlm.nih.gov/books/NBK10468/. Accessed Sept 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous