In vivo optical trapping indicates kinesin's stall force is reduced by dynein during intracellular transport
- PMID: 23404705
- PMCID: PMC3587256
- DOI: 10.1073/pnas.1219961110
In vivo optical trapping indicates kinesin's stall force is reduced by dynein during intracellular transport
Erratum in
- Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9613
Abstract
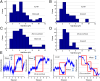
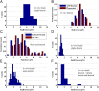
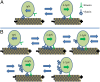
Kinesin and dynein are fundamental components of intracellular transport, but their interactions when simultaneously present on cargos are unknown. We built an optical trap that can be calibrated in vivo during data acquisition for each individual cargo to measure forces in living cells. Comparing directional stall forces in vivo and in vitro, we found evidence that cytoplasmic dynein is active during minus- and plus-end directed motion, whereas kinesin is only active in the plus direction. In vivo, we found outward (∼plus-end) stall forces range from 2 to 7 pN, which is significantly less than the 5- to 7-pN stall force measured in vitro for single kinesin molecules. In vitro measurements on beads with kinesin-1 and dynein bound revealed a similar distribution, implying that an interaction between opposite polarity motors causes this difference. Finally, inward (∼minus-end) stalls in vivo were 2-3 pN, which is higher than the 1.1-pN stall force of a single dynein, implying multiple active dynein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Schnitzer MJ, Block SM. Kinesin hydrolyses one ATP per 8-nm step. Nature. 1997;388(6640):386–390. - PubMed
-
- Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303(5658):676–678. - PubMed
-
- Kural C, et al. Kinesin and dynein move a peroxisome in vivo: A tug-of-war or coordinated movement? Science. 2005;308(5727):1469–1472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

