Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium
- PMID: 23404846
- PMCID: PMC3636525
- DOI: 10.1530/REP-12-0369
Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium
Abstract
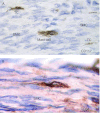
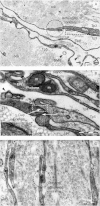
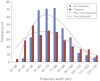
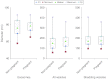
Telocytes (TCs) have been described in various organs and species (www.telocytes.com) as cells with telopodes (Tps) - very long cellular extensions with an alternation of thin segments (podomers) and dilated portions (podoms). We examined TCs using electron microscopy (EM), immunohistochemistry (IHC), immunofluorescence (IF), time-lapse videomicroscopy and whole-cell patch voltage clamp. EM showed a three-dimensional network of dichotomous-branching Tps, a labyrinthine system with homocellular and heterocellular junctions. Tps release extracellular vesicles (mean diameter of 160.6±6.9 nm in non-pregnant myometrium and 171.6±4.6 nm in pregnant myometrium), sending macromolecular signals to neighbouring cells. Comparative measurements (non-pregnant and pregnant myometrium) of podomer thickness revealed values of 81.94±1.77 vs 75.53±1.81 nm, while the podoms' diameters were 268.6±8.27 vs 316.38±17.56 nm. IHC as well as IF revealed double c-kit and CD34 positive results. Time-lapse videomicroscopy of cell culture showed dynamic interactions between Tps and myocytes. In non-pregnant myometrium, patch-clamp recordings of TCs revealed a hyperpolarisation-activated chloride inward current with calcium dependence and the absence of L-type calcium channels. TCs seem to have no excitable properties similar to the surrounding smooth muscle cells (SMCs). In conclusion, this study shows the presence of TCs as a distinct cell type in human non-pregnant and pregnant myometrium and describes morphometric differences between the two physiological states. In addition, we provide a preliminary in vitro electrophysiological evaluation of the non-pregnant state, suggesting that TCs could influence timing of the contractile activity of SMCs.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

