Cardiomyocyte ryanodine receptor degradation by chaperone-mediated autophagy
- PMID: 23404999
- PMCID: PMC3633160
- DOI: 10.1093/cvr/cvt029
Cardiomyocyte ryanodine receptor degradation by chaperone-mediated autophagy
Abstract
Aims: Chaperone-mediated autophagy (CMA) is a selective mechanism for the degradation of soluble cytosolic proteins bearing the sequence KFERQ. These proteins are targeted by chaperones and delivered to lysosomes where they are translocated into the lysosomal lumen and degraded via the lysosome-associated membrane protein type 2A (LAMP-2A). Mutations in LAMP2 that inhibit autophagy result in Danon disease characterized by hypertrophic cardiomyopathy. The ryanodine receptor type 2 (RyR2) plays a key role in cardiomyocyte excitation-contraction and its dysfunction can lead to cardiac failure. Whether RyR2 is degraded by CMA is unknown.
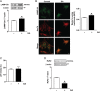
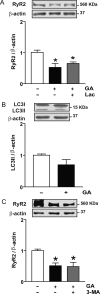
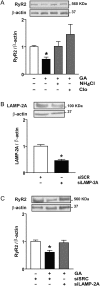
Methods and results: To induce CMA, cultured neonatal rat cardiomyocytes were treated with geldanamycin (GA) to promote protein degradation through this pathway. GA increased LAMP-2A levels together with its redistribution and colocalization with Hsc70 in the perinuclear region, changes indicative of CMA activation. The inhibition of lysosomes but not proteasomes prevented the loss of RyR2. The recovery of RyR2 content after incubation with GA by siRNA targeting LAMP-2A suggests that RyR2 is degraded via CMA. In silico analysis also revealed that the RyR2 sequence harbours six KFERQ motifs which are required for the recognition Hsc70 and its degradation via CMA. Our data suggest that presenilins are involved in RyR2 degradation by CMA.
Conclusion: These findings are consistent with a model in which oxidative damage of the RyR2 targets it for turnover by presenilins and CMA, which could lead to removal of damaged or leaky RyR2 channels.
Figures



References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2001;415:198–205. doi:10.1038/415198a. - DOI - PubMed
-
- Walsh LG, Tormey JMcD. Subcellular electrolyte shifts during in vitro myocardial ischemia and reperfusion. Am J Physiol. 1998;255:H917–H928. - PubMed
-
- Chohan PK, Singh RB, Dhalla NS, Netticadan T. L-arginine administration recovers sarcoplasmic reticulum function in ischemic reperfused hearts by preventing calpain activation. Cardiovasc Res. 2006;69:152–163. doi:10.1016/j.cardiores.2005.07.016. - DOI - PubMed
-
- Singh RB, Chohan PK, Dhalla NS, Netticadan T. The sarcoplasmic reticulum proteins are targets for calpain action in the ischemic-reperfused heart. J Mol Cell Cardiol. 2004;37:101–110. doi:10.1016/j.yjmcc.2004.04.009. - DOI - PubMed
-
- Takeda S, Mochizuki S, Saini HK, Elimban V, Dhalla NS. Modification of alterations in cardiac function and sarcoplasmic reticulum by vanadate in ischemic-reperfused rat hearts. J Appl Physiol. 2005;99:999–1005. doi:10.1152/japplphysiol.00234.2005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

