Monitoring the presence of ionic mercury in environmental water by plasmon-enhanced infrared spectroscopy
- PMID: 23405272
- PMCID: PMC3569543
- DOI: 10.1038/srep01175
Monitoring the presence of ionic mercury in environmental water by plasmon-enhanced infrared spectroscopy
Abstract
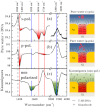
We demonstrate the ppt-level single-step selective monitoring of the presence of mercury ions (Hg²⁺) dissolved in environmental water by plasmon-enhanced vibrational spectroscopy. We combined a nanogap-optimized mid-infrared plasmonic structure with mercury-binding DNA aptamers to monitor in-situ the spectral evolution of the vibrational signal of the DNA induced by the mercury binding. Here, we adopted single-stranded thiolated 15-base DNA oligonucleotides that are immobilized on the Au surface and show strong specificity to Hg²⁺. The mercury-associated distinct signal is located apart from the biomolecule-associated broad signals and is selectively characterized. For example, with natural water from Lake Kasumigaura (Ibaraki Prefecture, Japan), direct detection of Hg²⁺ with a concentration as low as 37 ppt (37 × 10⁻¹⁰%) was readily demonstrated, indicating the high potential of this simple method for environmental and chemical sensing of metallic species in aqueous solution.
Figures




References
-
- Nolan E. M. & Lippard S. J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 108, 3443–3480 (2008). - PubMed
-
- Ministry of the environment, Japan. "Mercury analysis manual". National Institute for Minamata Disease website; http://www.nimd.go.jp/english/kenkyu/docs/2004_march_mercury_analysis_ma... Date of access: October32012.
-
- Cao R. G., Zhu B., Li J. & Xu D. Oligonucleotides-based biosensors with high sensitivity and selectivity for mercury using electrochemical impedance spectroscopy. Electrochem. Commun. 11, 1815–1818 (2009).
-
- Miyake Y., Togashi H., Tashiro M., Yamaguchi H., Oda S., Kudo M., Tanaka Y., Kondo Y., Sawa Y., Fujimoto T., Machinami T. & Ono A. MercuryII-mediated formation of thymine−HgII−thymine base pairs in DNA duplexes. J. Am. Chem. Soc. 128, 2172–2173 (2006). - PubMed
-
- Yang Y. -K., Yook, K.-J. & Tae, J. A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J. Am. Chem. Soc. 127, 16760–16761 (2005). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

