Chlorizidine, a cytotoxic 5H-pyrrolo[2,1-a]isoindol-5-one-containing alkaloid from a marine Streptomyces sp
- PMID: 23405849
- PMCID: PMC3702164
- DOI: 10.1021/ol303374e
Chlorizidine, a cytotoxic 5H-pyrrolo[2,1-a]isoindol-5-one-containing alkaloid from a marine Streptomyces sp
Abstract
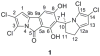
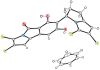
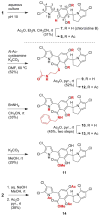
Cultivation of an obligate marine Streptomyces strain has provided the cytotoxic natural product chlorizidine A. X-ray crystallographic analysis revealed that the metabolite is composed of a chlorinated 2,3-dihydropyrrolizine ring attached to a chlorinated 5H-pyrrolo[2,1-a]isoindol-5-one. The carbon stereocenter in the dihydropyrrolizine is S-configured. Remarkably, the 5H-pyrrolo[2,1-a]isoindol-5-one moiety has no precedence in the field of natural products. The presence of this ring system, which was demonstrated to undergo facile nucleophilic substitution reactions at the activated carbonyl group, is essential to the molecule's cytotoxicity against HCT-116 human colon cancer cells.
Figures

References
-
-
Newman DJ, Cragg GM. J Nat Prod. 2012;75:311–335.. Butler MS. Nat Prod Rep. 2005;22:162–195.. For a microbial perspective, see: Berdy J. J Antibiot. 2005;58:1–26.. Demain AL, Sanchez S. J Antibiot. 2009;62:5–16.
-
-
- Waksman SA, Woodruff HB. Proc Soc Exper Biol Med. 1940;45:609–614.
- Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C. Biotechnol Bioeng. 1969;11:1101–1110. - PubMed
- Umezawa H, Maeda K, Takeuchi T, Okami Y. J Antibiot. 1966;19:200–209. - PubMed
- Hata T, Koga F, Sano Y, Kanamori K, Matsumae A, Sugawara R, Hoshi T, Shima T, Ito S, Tomizawa S. J Antibiot. 1954;7:107–112. - PubMed
- Sato K, Okamura N, Utagawa K, Ito Y, Watanabe M. Sci Rep Res Inst Tohoku Univ Med. 1960;9:224–232. - PubMed
- Julian PE, Schenck JR. Antibiotics and Chemotherapy. 1953;3:1218–1220. - PubMed
- Hata T, Hoshi T, Kanamori K, Matsumae A, Sano Y, Shima T, Sugawara R. J Antibiot. 1956;9:141–146. - PubMed
- Umezawa H, Takeuchi T, Nitta K, Yamamoto T, Yamaoka S. J Antibiot. 1953;6:101. - PubMed
- Vavra JJ, Deboer C, Dietz A, Hanka LJ, Sokolski WT. Antibiot Annu. 1959;7:230–235. - PubMed
- Ishida N, Miyazaki K, Kumagai K, Rikimaru M. J Antibiot. 1965;18:68–76. - PubMed
-
- Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Angew Chem Int Ed. 2003;42:355–357. - PubMed
-
-
Strain CNH-287 (GenBank accession no. bankit JX680598) was obtained from a marine sediment sample collected at the intertidal zone near San Clemente, CA.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

