Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets
- PMID: 23405892
- PMCID: PMC3578301
- DOI: 10.1111/imr.12032
Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets
Erratum in
- Immunol Rev. 2013 Sep;255(1):275
Abstract
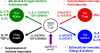
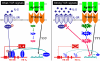
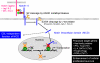
CD4(+) T-helper (Th) cells are a major cell population that play an important role in governing acquired immune responses to a variety of foreign antigens as well as inducing some types of autoimmune diseases. There are at least four distinct Th cell subsets (Th1, Th2, Th17, and inducible T-regulatory cells), each of which has specialized functions to control immune responses. Each of these cell types emerge from naive CD4(+) T cells after encounter with foreign antigens presented by dendritic cells (DCs). Each Th cell subset expresses a unique set of transcription factors and produces hallmark cytokines. Both T-cell receptor (TCR)-mediated stimulation and the cytokine environment created by activated CD4(+) T cells themselves, by 'partner' DCs, and/or other cell types during the course of differentiation, play an important role in the fate decisions toward distinct Th subsets. Here, we review how TCR-mediated signals in collaboration with the cytokine environment influence the fate decisions of naive CD4(+) T cells toward distinct Th subsets at the early stages of activation. We also discuss the roles of TCR-proximal signaling intermediates and of the Notch pathway in regulating the differentiation to distinct Th phenotypes.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures

References
-
- Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. - PubMed
-
- Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158:4237–4244. - PubMed
-
- Jorritsma PJ, Brogdon JL, Bottomly K. Role of TCR-induced extracellular signal-regulated kinase activation in the regulation of early IL-4 expression in naive CD4+ T cells. J Immunol. 2003;170:2427–2434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

