Metabolic control of the Treg/Th17 axis
- PMID: 23405895
- PMCID: PMC3576873
- DOI: 10.1111/imr.12029
Metabolic control of the Treg/Th17 axis
Abstract
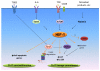
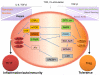
The interplay of the immune system with other aspects of physiology is continually being revealed and in some cases studied in considerable mechanistic detail. A prime example is the influence of metabolic cues on immune responses. It is well appreciated that upon activation, T cells take on a metabolic profile profoundly distinct from that of their quiescent and anergic counterparts; however, a number of recent breakthroughs have greatly expanded our knowledge of how aspects of cellular metabolism can shape a T-cell response. Particularly important are findings that certain environmental cues can tilt the delicate balance between inflammation and immune tolerance by skewing T-cell fate decisions toward either the T-helper 17 (Th17) or T-regulatory (Treg) cell lineage. Recognizing the unappreciated immune-modifying potential of metabolic factors and particularly those involved in the generation of these functionally opposing T-cell subsets will likely add new and potent therapies to our repertoire for treating immune mediated pathologies. In this review, we summarize and discuss recent findings linking certain metabolic pathways, enzymes, and by-products to shifts in the balance between Th17 and Treg cell populations. These advances highlight numerous opportunities for immune modulation.
© 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd.
Figures
References
-
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. - PubMed
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. - PubMed
-
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

