The Th17 family: flexibility follows function
- PMID: 23405897
- PMCID: PMC3607325
- DOI: 10.1111/imr.12035
The Th17 family: flexibility follows function
Abstract
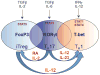
Discovery of the T-helper 17 (Th17) subset heralded a major shift in T-cell biology and immune regulation. In addition to defining a new arm of the adaptive immune response, studies of the Th17 pathway have led to a greater appreciation of the developmental flexibility, or plasticity, that is a feature of T-cell developmental programs. Since the initial finding that differentiation of Th17 cells is promoted by transforming growth factor-β (TGFβ), it became clear that Th17 cell development overlapped that of induced regulatory T (iTreg) cells. Subsequent findings established that Th17 cells are also unusually flexible in their late developmental programming, demonstrating substantial overlap with conventional Th1 cells through mechanisms that are just beginning to be understood but would appear to have important implications for immunoregulation at homeostasis and in immune-mediated diseases. Herein we examine the developmental and functional features of Th17 cells in relation to iTreg cells, Th1 cells, and Th22 cells, as a basis for understanding the contributions of this pathway to host defense, immune homeostasis, and immune-mediated disease.
© 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

