Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer
- PMID: 23406027
- PMCID: PMC3615415
- DOI: 10.1056/NEJMoa1209288
Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer
Abstract
Background: Metastatic thyroid cancers that are refractory to radioiodine (iodine-131) are associated with a poor prognosis. In mouse models of thyroid cancer, selective mitogen-activated protein kinase (MAPK) pathway antagonists increase the expression of the sodium-iodide symporter and uptake of iodine. Their effects in humans are not known.
Methods: We conducted a study to determine whether the MAPK kinase (MEK) 1 and MEK2 inhibitor selumetinib (AZD6244, ARRY-142886) could reverse refractoriness to radioiodine in patients with metastatic thyroid cancer. After stimulation with thyrotropin alfa, dosimetry with iodine-124 positron-emission tomography (PET) was performed before and 4 weeks after treatment with selumetinib (75 mg twice daily). If the second iodine-124 PET study indicated that a dose of iodine-131 of 2000 cGy or more could be delivered to the metastatic lesion or lesions, therapeutic radioiodine was administered while the patient was receiving selumetinib.
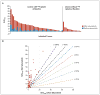
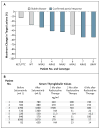
Results: Of 24 patients screened for the study, 20 could be evaluated. The median age was 61 years (range, 44 to 77), and 11 patients were men. Nine patients had tumors with BRAF mutations, and 5 patients had tumors with mutations of NRAS. Selumetinib increased the uptake of iodine-124 in 12 of the 20 patients (4 of 9 patients with BRAF mutations and 5 of 5 patients with NRAS mutations). Eight of these 12 patients reached the dosimetry threshold for radioiodine therapy, including all 5 patients with NRAS mutations. Of the 8 patients treated with radioiodine, 5 had confirmed partial responses and 3 had stable disease; all patients had decreases in serum thyroglobulin levels (mean reduction, 89%). No toxic effects of grade 3 or higher attributable by the investigators to selumetinib were observed. One patient received a diagnosis of myelodysplastic syndrome more than 51 weeks after radioiodine treatment, with progression to acute leukemia.
Conclusions: Selumetinib produces clinically meaningful increases in iodine uptake and retention in a subgroup of patients with thyroid cancer that is refractory to radioiodine; the effectiveness may be greater in patients with RAS-mutant disease. (Funded by the American Thyroid Association and others; ClinicalTrials.gov number, NCT00970359.).
Figures

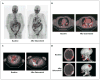
Comment in
-
Selumetinib promotes radioiodine uptake in thyroid cancers.Nat Rev Endocrinol. 2013 May;9(5):253. doi: 10.1038/nrendo.2013.48. Epub 2013 Mar 5. Nat Rev Endocrinol. 2013. PMID: 23458781 No abstract available.
References
-
- Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–63. - PubMed
-
- Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9. - PubMed
-
- Coelho SM, Corbo R, Buescu A, Carvalho DP, Vaisman M. Retinoic acid in patients with radioiodine non-responsive thyroid carcinoma. J Endocrinol Invest. 2004;27:334–9. - PubMed
-
- Simon D, Körber C, Krausch M, et al. Clinical impact of retinoids in redifferentiation therapy of advanced thyroid cancer: final results of a pilot study. Eur J Nucl Med Mol Imaging. 2002;29:775–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
