Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein
- PMID: 23406369
- PMCID: PMC3638559
- DOI: 10.1089/ten.TEA.2012.0367
Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein
Abstract
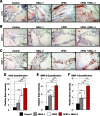
An ideal mesenchymal stem cell (MSC) source for bone tissue engineering has yet to be identified. Such an MSC population would be easily harvested in abundance, with minimal morbidity and with high purity. Our laboratories have identified perivascular stem cells (PSCs) as a candidate cell source. PSCs are readily isolatable through fluorescent-activated cell sorting from adipose tissue and have been previously shown to be indistinguishable from MSCs in the phenotype and differentiation potential. PSCs consist of two distinct cell populations: (1) pericytes (CD146+, CD34-, and CD45-), which surround capillaries and microvessels, and (2) adventitial cells (CD146-, CD34+, and CD45-), found within the tunica adventitia of large arteries and veins. We previously demonstrated the osteogenic potential of pericytes by examining pericytes derived from the human fetal pancreas, and illustrated their in vivo trophic and angiogenic effects. In the present study, we used an intramuscular ectopic bone model to develop the translational potential of our original findings using PSCs (as a combination of pericytes and adventitial cells) from human white adipose tissue. We evaluated human PSC (hPSC)-mediated bone formation and vascularization in vivo. We also examined the effects of hPSCs when combined with the novel craniosynostosis-associated protein, Nel-like molecule I (NELL-1). Implants consisting of the demineralized bone matrix putty combined with NELL-1 (3 μg/μL), hPSC (2.5×10(5) cells), or hPSC+NELL-1, were inserted in the bicep femoris of SCID mice. Bone growth was evaluated using microcomputed tomography, histology, and immunohistochemistry over 4 weeks. Results demonstrated the osteogenic potential of hPSCs and the additive effect of hPSC+NELL-1 on bone formation and vasculogenesis. Comparable osteogenesis was observed with NELL-1 as compared to the more commonly used bone morphogenetic protein-2. Next, hPSCs induced greater implant vascularization than the unsorted stromal vascular fraction from patient-matched samples. Finally, we observed an additive effect on implant vascularization with hPSC+NELL-1 by histomorphometry and immunohistochemistry, accompanied by in vitro elaboration of vasculogenic growth factors. These findings hold significant implications for the cell/protein combination therapy hPSC+NELL-1 in the development of strategies for vascularized bone regeneration.
Figures





References
-
- Giannoudis P.V. Dinopoulos H. Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20. - PubMed
-
- Laurencin C.T. Ambrosio A.M. Borden M.D. Cooper J.A., Jr. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19. - PubMed
-
- Laurie S.W. Kaban L.B. Mulliken J.B. Murray J.E. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933. - PubMed
-
- Frodel J.L., Jr. Marentette L.J. Quatela V.C. Weinstein G.S. Calvarial bone graft harvest. Techniques, considerations, and morbidity. Arch Otolaryngol Head Neck Surg. 1993;119:17. - PubMed
-
- Cancedda R. Mastrogiacomo M. Bianchi G. Derubeis A. Muraglia A. Quarto R. Bone marrow stromal cells and their use in regenerating bone. Novartis Found Symp. 2003;249:133. discussion 143, 170, 239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

