Dynamic shear stress regulation of inflammatory and thrombotic pathways in baboon endothelial outgrowth cells
- PMID: 23406430
- PMCID: PMC3665318
- DOI: 10.1089/ten.TEA.2012.0300
Dynamic shear stress regulation of inflammatory and thrombotic pathways in baboon endothelial outgrowth cells
Abstract
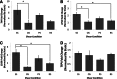
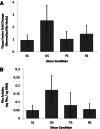
Endothelial outgrowth cells (EOCs) have garnered much attention as a potential autologous endothelial source for vascular implants or in tissue engineering applications due to their ease of isolation and proliferative ability; however, how these cells respond to different hemodynamic cues is ill-defined. This study investigates the inflammatory and thrombotic response of baboon EOCs (BaEOCs) to four hemodynamic conditions using the cone and plate shear apparatus: steady, laminar shear stress (SS); pulsatile, nonreversing laminar shear stress (PS); oscillatory, laminar shear stress (OS); and net positive, pulsatile, reversing laminar shear stress (RS). In summary, endothelial nitric oxide synthase (eNOS) mRNA was significantly upregulated by SS compared to OS. No differences were found in the mRNA levels of the inflammatory markers intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1) between the shear conditions; however, OS significantly increased the number of monocytes bound when compared to SS. Next, SS increased the anti-thrombogenic mRNA levels of CD39, thrombomodulin, and endothelial protein-C receptor (EPCR) compared to OS. SS also significantly increased CD39 and EPCR mRNA levels compared to RS. Finally, no significant differences were detected when comparing pro-thrombotic tissue factor mRNA or its activity levels. These results indicate that shear stress can have beneficial (SS) or adverse (OS, RS) effects on the inflammatory or thrombotic potential of EOCs. Further, these results suggest SS hemodynamic preconditioning may be optimal in increasing the efficacy of a vascular implant or in tissue-engineered applications that have incorporated EOCs.
Figures





References
-
- Hinds M.T. Ma M. Tran N. Ensley A.E. Kladakis S.M. Vartanian K.B., et al. Potential of baboon endothelial progenitor cells for tissue engineered vascular grafts. J Biomed Mater Res Part A. 2008;86A:804. - PubMed
-
- Griese D.P. Ehsan A. Melo L.G. Kong D. Zhang L. Mann M.J., et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts. Circulation. 2003;108:2710. - PubMed
-
- Tao J. Yang Z. Wang J.M. Tu C. Pan S.R. Effects of fluid shear stress on eNOS mRNA expression and NO production in human endothelial progenitor cells. Cardiology. 2006;106:82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

