Conditional knockdown of DNA methyltransferase 1 reveals a key role of retinal pigment epithelium integrity in photoreceptor outer segment morphogenesis
- PMID: 23406904
- PMCID: PMC3585665
- DOI: 10.1242/dev.086603
Conditional knockdown of DNA methyltransferase 1 reveals a key role of retinal pigment epithelium integrity in photoreceptor outer segment morphogenesis
Abstract
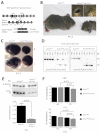
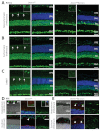
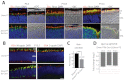
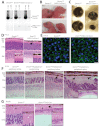
Dysfunction or death of photoreceptors is the primary cause of vision loss in retinal and macular degenerative diseases. As photoreceptors have an intimate relationship with the retinal pigment epithelium (RPE) for exchange of macromolecules, removal of shed membrane discs and retinoid recycling, an improved understanding of the development of the photoreceptor-RPE complex will allow better design of gene- and cell-based therapies. To explore the epigenetic contribution to retinal development we generated conditional knockout alleles of DNA methyltransferase 1 (Dnmt1) in mice. Conditional Dnmt1 knockdown in early eye development mediated by Rx-Cre did not produce lamination or cell fate defects, except in cones; however, the photoreceptors completely lacked outer segments despite near normal expression of phototransduction and cilia genes. We also identified disruption of RPE morphology and polarization as early as E15.5. Defects in outer segment biogenesis were evident with Dnmt1 exon excision only in RPE, but not when excision was directed exclusively to photoreceptors. We detected a reduction in DNA methylation of LINE1 elements (a measure of global DNA methylation) in developing mutant RPE as compared with neural retina, and of Tuba3a, which exhibited dramatically increased expression in mutant retina. These results demonstrate a unique function of DNMT1-mediated DNA methylation in controlling RPE apicobasal polarity and neural retina differentiation. We also establish a model to study the epigenetic mechanisms and signaling pathways that guide the modulation of photoreceptor outer segment morphogenesis by RPE during retinal development and disease.
Figures


Similar articles
-
Dnmt1, Dnmt3a and Dnmt3b cooperate in photoreceptor and outer plexiform layer development in the mammalian retina.Exp Eye Res. 2017 Jun;159:132-146. doi: 10.1016/j.exer.2016.11.014. Epub 2016 Nov 16. Exp Eye Res. 2017. PMID: 27865785
-
Proteomic analysis of the retina: removal of RPE alters outer segment assembly and retinal protein expression.Glia. 2009 Mar;57(4):380-92. doi: 10.1002/glia.20765. Glia. 2009. PMID: 18803304 Free PMC article.
-
Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal.J Neurosci. 2000 Oct 1;20(19):7149-57. doi: 10.1523/JNEUROSCI.20-19-07149.2000. J Neurosci. 2000. PMID: 11007870 Free PMC article.
-
Mechanism of Photoreceptor Outer Segment Tip Ingestion: Evidence of Trogocytosis.Adv Exp Med Biol. 2025;1468:309-311. doi: 10.1007/978-3-031-76550-6_51. Adv Exp Med Biol. 2025. PMID: 39930214 Review.
-
Phagocytosis by the retinal pigment epithelium: New insights into polarized cell mechanics.Bioessays. 2025 Jan;47(1):e2300197. doi: 10.1002/bies.202300197. Epub 2024 Dec 11. Bioessays. 2025. PMID: 39663766 Free PMC article. Review.
Cited by
-
Comparison of Developmental Dynamics in Human Fetal Retina and Human Pluripotent Stem Cell-Derived Retinal Tissue.Stem Cells Dev. 2021 Apr;30(8):399-417. doi: 10.1089/scd.2020.0085. Stem Cells Dev. 2021. PMID: 33677999 Free PMC article.
-
Retinal Cell Transplantation, Biomaterials, and In Vitro Models for Developing Next-generation Therapies of Age-related Macular Degeneration.Stem Cells Transl Med. 2022 Mar 31;11(3):269-281. doi: 10.1093/stcltm/szac001. Stem Cells Transl Med. 2022. PMID: 35356975 Free PMC article. Review.
-
Efficient induction of productive Cre-mediated recombination in retinal pigment epithelium.Mol Vis. 2014 Apr 11;20:480-7. eCollection 2014. Mol Vis. 2014. PMID: 24744608 Free PMC article.
-
The role of epigenetic methylation/demethylation in the regulation of retinal photoreceptors.Front Cell Dev Biol. 2023 May 26;11:1149132. doi: 10.3389/fcell.2023.1149132. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37305686 Free PMC article. Review.
-
Accelerated and Improved Differentiation of Retinal Organoids from Pluripotent Stem Cells in Rotating-Wall Vessel Bioreactors.Stem Cell Reports. 2018 Jan 9;10(1):300-313. doi: 10.1016/j.stemcr.2017.11.001. Epub 2017 Dec 7. Stem Cell Reports. 2018. PMID: 29233554 Free PMC article.
References
-
- Arikawa K., Williams D. S. (1993). Acetylated alpha-tubulin in the connecting cilium of developing rat photoreceptors. Invest. Ophthalmol. Vis. Sci. 34, 2145–2149 - PubMed
-
- Bäumer N., Marquardt T., Stoykova A., Ashery-Padan R., Chowdhury K., Gruss P. (2002). Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development 129, 4535–4545 - PubMed
-
- Bernstein B. E., Meissner A., Lander E. S. (2007). The mammalian epigenome. Cell 128, 669–681 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

