Regulatory effect of calcineurin inhibitor, tacrolimus, on IL-6/sIL-6R-mediated RANKL expression through JAK2-STAT3-SOCS3 signaling pathway in fibroblast-like synoviocytes
- PMID: 23406906
- PMCID: PMC3672788
- DOI: 10.1186/ar4162
Regulatory effect of calcineurin inhibitor, tacrolimus, on IL-6/sIL-6R-mediated RANKL expression through JAK2-STAT3-SOCS3 signaling pathway in fibroblast-like synoviocytes
Abstract
Introduction: This study investigated whether the calcineurin inhibitor, tacrolimus, suppresses receptor activator of NF-κB ligand (RANKL) expression in fibroblast-like synoviocytes (FLS) through regulation of IL-6/Janus activated kinase (JAK2)/signal transducer and activator of transcription-3 (STAT3) and suppressor of cytokine signaling (SOCS3) signaling.
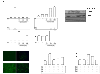
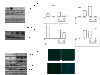
Methods: The expression of RANKL, JAK2, STAT3, and SOCS3 proteins was assessed by western blot analysis, real-time PCR and ELISA in IL-6 combined with soluble IL-6 receptor (sIL-6R)-stimulated rheumatoid arthritis (RA)-FLS with or without tacrolimus treatment. The effects of tacrolimus on synovial inflammation and bone erosion were assessed using mice with arthritis induced by K/BxN serum. Immunofluorescent staining was performed to identify the effect of tacrolimus on RANKL and SOCS3. The tartrate-resistant acid phosphatase staining assay was performed to assess the effect of tacrolimus on osteoclast differentiation.
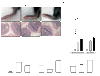
Results: We found that RANKL expression in RA FLS is regulated by the IL-6/sIL-6R/JAK2/STAT3/SOCS3 pathway. Inhibitory effects of tacrolimus on RANKL expression in a serum-induced arthritis mice model were identified. Tacrolimus inhibits RANKL expression in IL-6/sIL-6R-stimulated FLS by suppressing STAT3. Among negative regulators of the JAK/STAT pathway, such as CIS1, SOCS1, and SOCS3, only SOCS3 is significantly induced by tacrolimus. As compared to dexamethasone and methotrexate, tacrolimus more potently suppresses RANKL expression in FLS. By up-regulating SOCS3, tacrolimus down-regulates activation of the JAK-STAT pathway by IL-6/sIL-6R trans-signaling, thus decreasing RANKL expression in FLS.
Conclusions: These data suggest that tacrolimus might affect the RANKL expression in IL-6 stimulated FLS through STAT3 suppression, together with up-regulation of SOCS3.
Figures

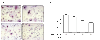

Similar articles
-
Combination of 4-hydroperoxy cyclophosphamide and methotrexate inhibits IL-6/sIL-6R-induced RANKL expression in fibroblast-like synoviocytes via suppression of the JAK2/STAT3 and p38MAPK signaling pathway.Int Immunopharmacol. 2018 Aug;61:45-53. doi: 10.1016/j.intimp.2018.05.014. Epub 2018 May 24. Int Immunopharmacol. 2018. PMID: 29803913
-
IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17.Rheumatology (Oxford). 2008 Nov;47(11):1635-40. doi: 10.1093/rheumatology/ken363. Epub 2008 Sep 11. Rheumatology (Oxford). 2008. PMID: 18786965
-
An herbal formula inhibits STAT3 signaling and attenuates bone erosion in collagen-induced arthritis rats.Phytomedicine. 2020 Sep;76:153254. doi: 10.1016/j.phymed.2020.153254. Epub 2020 May 30. Phytomedicine. 2020. PMID: 32531698
-
Interleukin 27 Signaling in Rheumatoid Arthritis Patients: Good or Evil?Front Immunol. 2022 Jan 4;12:787252. doi: 10.3389/fimmu.2021.787252. eCollection 2021. Front Immunol. 2022. PMID: 35058928 Free PMC article. Review.
-
Inhibition of IL-6 family cytokines by SOCS3.Semin Immunol. 2014 Feb;26(1):13-9. doi: 10.1016/j.smim.2013.12.004. Epub 2014 Jan 10. Semin Immunol. 2014. PMID: 24418198 Free PMC article. Review.
Cited by
-
Role of suppressors of cytokine signaling 3 in bone inflammatory responses.Front Immunol. 2014 Jan 10;4:506. doi: 10.3389/fimmu.2013.00506. eCollection 2014 Jan 10. Front Immunol. 2014. PMID: 24454312 Free PMC article. Review.
-
Regulatory effect of anti-gp130 functional mAb on IL-6 mediated RANKL and Wnt5a expression through JAK-STAT3 signaling pathway in FLS.Oncotarget. 2018 Jan 4;9(29):20366-20376. doi: 10.18632/oncotarget.23917. eCollection 2018 Apr 17. Oncotarget. 2018. PMID: 29755657 Free PMC article.
-
Tacrolimus Modulates TGF-β Signaling-Related Genes and MicroRNAs in Human Retinal Pigment Epithelial Cells Activated by Lipopolysaccharide.Int J Mol Sci. 2025 Jun 4;26(11):5402. doi: 10.3390/ijms26115402. Int J Mol Sci. 2025. PMID: 40508210 Free PMC article.
-
K/BxN Serum-Transfer Arthritis as a Model for Human Inflammatory Arthritis.Front Immunol. 2016 Jun 2;7:213. doi: 10.3389/fimmu.2016.00213. eCollection 2016. Front Immunol. 2016. PMID: 27313578 Free PMC article. Review.
-
Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis.Theranostics. 2019 Aug 14;9(22):6424-6442. doi: 10.7150/thno.35528. eCollection 2019. Theranostics. 2019. PMID: 31588227 Free PMC article. Review.
References
-
- Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol. 1999;163:434–442. - PubMed
-
- Crotti TN, Smith MD, Weedon H, Ahern MJ, Findlay DM, Kraan M, Tak PP, Haynes DR. Receptor activator NF-κB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61:1047–1054. doi: 10.1136/ard.61.12.1047. - DOI - PMC - PubMed
-
- Ainola M, Mandelin J, Liljeström M, Konttinen YT, Salo J. Imbalanced expression of RANKL and osteoprotegerin mRNA in pannus tissue of rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:240–246. - PubMed
-
- Tunyogi-Csapo M, Kis-Toth K, Radacs M, Farkas B, Jacobs JJ, Finnegan A, Mikecz K, Glant TT. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: fibroblast-mediated pathologic bone resorption. Arthritis Rheum. 2008;58:2397–2408. doi: 10.1002/art.23653. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

