The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation
- PMID: 23406974
- PMCID: PMC3600839
- DOI: 10.1093/humrep/des467
The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation
Abstract
Study question: What is the prevalence of defects in the Ca(2+)-signalling pathways mediating hyperactivation (calcium influx and store mobilization) among donors and sub-fertile patients and are they functionally significant, i.e. related to fertilization success at IVF?
Summary answer: This study identifies, for the first time, the prevalence of Ca(2+) store defects in sperm from research donors, IVF and ICSI patients. It highlights the biological role and importance of Ca(2+) signalling (Ca(2+) store mobilization) for fertilization at IVF.
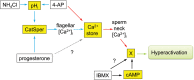
What is known already: Sperm motility and hyperactivation (HA) are important for fertility, mice with sperm incapable of HA are sterile. Recently, there has been significant progress in our knowledge of the factors controlling these events, in particular the generation and regulation of calcium signals. Both pH-regulated membrane Ca(2+) channels (CatSper) and Ca(2+) stores (potentially activating store-operated Ca(2+) channels) have been implicated in controlling HA.
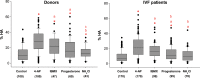
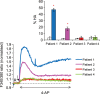
Study design, size, and duration: This was a prospective study examining a panel of 68 donors and 181 sub-fertile patients attending the Assisted Conception Unit, Ninewells Hospital Dundee for IVF and ICSI. Twenty-five of the donors gave a second sample (∼4 weeks later) to confirm consistency/reliability of the recorded responses. Ca(2+) signalling was manipulated using three agonists, NH4Cl (activates CatSper via pH), progesterone (direct activation of CatSper channels, potentially enhancing mobilization of stored Ca(2+) by CICR) and 4-aminopyridine (4-AP) (effect on pH equivalent to NH4Cl and mobilizes stored Ca(2+)). The broad-spectrum phosphodiesterase inhibitor 3-isobutyl-1-methyxanthine (IBMX), a potent activator of HA was also used for comparison. For patient samples, an aliquot surplus to requirements for IVF/ICSI treatment was examined, allowing direct comparison of Ca(2+) signalling and motility data with functional competence of the sperm.
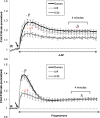
Materials, setting, methods: The donors and sub-fertile patients were screened for HA (using CASA) and changes in intracellular Ca(2+) were assessed by loading with Fura-2 and measuring fluorescence using a plate reader (FluoStar).
Main results and the role of chance: The relative efficacy of the stimuli in inducing HA was 4-AP >> IBMX > progesterone. NH4Cl increased [Ca(2+)]i similarly to 4-AP and progesterone but did not induce a significant increase in HA. Failure of samples to generate HA (no significant increase in response to stimulation with 4-AP) was seen in just 2% of research donors but occurred in 10% of IVF patients (P = 0.025). All donor samples generated a significant [Ca(2+)]i increase when stimulated with 4-AP but 3.3% of IVF and 28.6% of ICSI patients failed to respond. Amplitudes of HA and [Ca(2+)]i responses to 4-AP were correlated with fertilization rate at IVF (P= 0.029; P = 0.031, respectively). Progesterone reliably induced [Ca(2+)]i responses (97% of donors, 100% of IVF patients) but was significantly less effective than 4-AP in inducing HA. Twenty seven per cent of ICSI patients failed to generate a [Ca(2+)]i response to progesterone (P= 0.035). Progesterone-induced [Ca(2+)]i responses were correlated with fertilization rate at IVF (P= 0.037) but induction of HA was not. In donor samples examined on more than one occasion consistent responses for 4-AP-induced [Ca(2+)]i (R(2) = 0.97) and HA (R(2) = 0.579) were obtained. In summary, the data indicate that defects in Ca(2+) signalling leading to poor HA do occur and that ability to undergo Ca(2+) -induced HA affects IVF fertilizing capacity. The data also confirm that release of stored Ca(2+) is the crucial component of Ca(2+) signals leading to HA and that Ca(2+) store defects may therefore underlie HA failure.
Limitations, reasons for caution: This is an in vitro study of sperm function. While the repeatability of the [Ca(2+)]i and HA responses in samples from the same donor were confirmed, data for patients were from 1 assessment and thus the robustness of the failed responses in patients' needs to be established. The focus of this study was on using 4AP, which mobilizes stored Ca(2+) and is a potent inducer of HA. The n values for other agonists, especially calcium assessments, are smaller.
Wider implications of the findings: Previous studies have shown a significant relationship between basal levels of HA, calcium responses to progesterone and IVF fertilization rates. Here, we have systematically investigated the ability/failure of human sperm to generate Ca(2+) signals and HA in response to targeted pharmacological challenge and, related defects in these responses to IVF success. [Ca(2+)]i signalling is fundamental for sperm motility and data from this study will lead to assessment of the nature of these defects using techniques such as single-cell imaging and patch clamping.
Study funding/competing interest(s): Resources from a Wellcome Trust Project Grant (#086470, Publicover and Barratt PI) primarily funded the study. The authors have no competing interests.
Figures

References
-
- Aitken RJ, Irvine DS, Wu FC. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol. 1991;164:542–551. - PubMed
-
- Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, Thulliez M, Borot N, Moati L, Barthelme A, Shalmon L, et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet. 2003;11:497–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

