Beneficial effects of 20(S)-protopanaxadiol on antitumor activity and toxicity of cyclophosphamide in tumor-bearing mice
- PMID: 23407364
- PMCID: PMC3570184
- DOI: 10.3892/etm.2012.820
Beneficial effects of 20(S)-protopanaxadiol on antitumor activity and toxicity of cyclophosphamide in tumor-bearing mice
Abstract
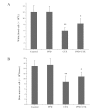
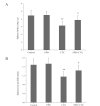
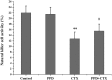
20(S)-protopanaxadiol (PPD) is an extract of Panax quinquefolius L. The aim of this study was to investigate the effect of PPD on the antitumor activity and toxicity of cyclophosphamide (CTX) in tumor-bearing mice. C57BL/6 mice bearing Lewis lung carcinoma cells were treated with PPD (50 mg/kg) alone, CTX (20 mg/kg) alone or PPD (50 mg/kg) in combination with CTX (20 mg/kg), respectively. The results showed that PPD alone has no significant antitumor activity but synergistically enhanced the antitumor activity of CTX. PPD significantly increased the peripheral white blood cell count, bone marrow cell count, interleukin-2 and interferon-γ in CTX-treated tumor-bearing mice. The lowered levels of spleen index, splenocyte proliferation and natural killer cell activity in tumor-bearing mice following CTX treatment were also increased by PPD administration. PPD may be a beneficial supplement during CTX chemotherapy for enhancing the antitumor efficacy and reducing the toxicity of CTX.
Keywords: 20(S)-protopanaxadiol; cyclophosphamide; spleen index; white blood cell count.
Figures

Similar articles
-
Immune regulation effect of lienal polypeptides extract in Lewis lung carcinoma-bearing mice treated with cyclophosphamide.Exp Biol Med (Maywood). 2018 Jan;243(1):66-77. doi: 10.1177/1535370217737982. Epub 2017 Oct 27. Exp Biol Med (Maywood). 2018. PMID: 29078731 Free PMC article.
-
Protective effect of triterpenoid fractions from the rhizomes of Astilbe chinensis on cyclophosphamide-induced toxicity in tumor-bearing mice.J Ethnopharmacol. 2008 Sep 26;119(2):312-7. doi: 10.1016/j.jep.2008.07.017. Epub 2008 Jul 23. J Ethnopharmacol. 2008. PMID: 18692125
-
Neutral polysaccharide from Panax notoginseng enhanced cyclophosphamide antitumor efficacy in hepatoma H22-bearing mice.BMC Cancer. 2021 Jan 7;21(1):37. doi: 10.1186/s12885-020-07742-z. BMC Cancer. 2021. PMID: 33413214 Free PMC article.
-
Synergistic Antitumor Effect of Grifola frondose Polysaccharide-Protein Complex in Combination with Cyclophosphamide in H22 Tumor-Bearing Mice.Molecules. 2023 Mar 26;28(7):2954. doi: 10.3390/molecules28072954. Molecules. 2023. PMID: 37049720 Free PMC article.
-
Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice.J Clin Invest. 1998 Jan 15;101(2):429-41. doi: 10.1172/JCI1348. J Clin Invest. 1998. PMID: 9435316 Free PMC article.
Cited by
-
Immune regulation effect of lienal polypeptides extract in Lewis lung carcinoma-bearing mice treated with cyclophosphamide.Exp Biol Med (Maywood). 2018 Jan;243(1):66-77. doi: 10.1177/1535370217737982. Epub 2017 Oct 27. Exp Biol Med (Maywood). 2018. PMID: 29078731 Free PMC article.
-
Ginsenoside Rg3 induces ginsenoside Rb1-comparable cardioprotective effects independent of reducing blood pressure in spontaneously hypertensive rats.Exp Ther Med. 2017 Nov;14(5):4977-4985. doi: 10.3892/etm.2017.5198. Epub 2017 Sep 22. Exp Ther Med. 2017. PMID: 29201202 Free PMC article.
-
Protopanaxadiol manipulates gut microbiota to promote bone marrow hematopoiesis and enhance immunity in cyclophosphamide-induced immunosuppression mice.MedComm (2020). 2023 Feb 23;4(2):e222. doi: 10.1002/mco2.222. eCollection 2023 Apr. MedComm (2020). 2023. PMID: 36845073 Free PMC article.
-
Ginsenoside-Rb3 protects the myocardium from ischemia-reperfusion injury via the inhibition of apoptosis in rats.Exp Ther Med. 2014 Dec;8(6):1751-1756. doi: 10.3892/etm.2014.2007. Epub 2014 Oct 7. Exp Ther Med. 2014. PMID: 25371727 Free PMC article.
-
Platelet Protease Activated Receptor 1 Is Involved in the Hemostatic Effect of 20(S)-Protopanaxadiol by Regulating Calcium Signaling.Front Pharmacol. 2020 Sep 18;11:549150. doi: 10.3389/fphar.2020.549150. eCollection 2020. Front Pharmacol. 2020. PMID: 33041793 Free PMC article.
References
-
- Pass GJ, Carrie D, Boylan M, et al. Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome p450 reductase null mouse. Cancer Res. 2005;65:4211–4217. - PubMed
-
- Papaldo P, Lopez M, Marolla P, et al. Impact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamide. J Clin Oncol. 2005;23:6908–6918. - PubMed
-
- Pratheeshkumar P, Kuttan G. Ameliorative action of Vernonia cinerea L. on cyclophosphamide-induced immunosuppression and oxidative stress in mice. Inflammopharmacology. 2010;18:197–207. - PubMed
-
- Wang T, Yu X, Qu S, Xu H, Han B, Sui D. Effect of ginsenoside Rb3 on myocardial injury and heart function impairment induced by isoproterenol in rats. Eur J Pharmacol. 2010;636:121–125. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
