Manifestations of immune tolerance in the human female reproductive tract
- PMID: 23407606
- PMCID: PMC3570961
- DOI: 10.3389/fimmu.2013.00026
Manifestations of immune tolerance in the human female reproductive tract
Abstract
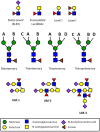
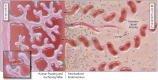
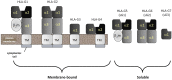
Like other mucosal surfaces (e.g., the gastrointestinal tract, the respiratory tract), the human female reproductive tract acts as an initial barrier to foreign antigens. In this role, the epithelial surface and subepithelial immune cells must balance protection against pathogenic insults against harmful inflammatory reactions and acceptance of particular foreign antigens. Two common examples of these acceptable foreign antigens are the fetal allograft and human semen/sperm. Both are purposely deposited into the female genital tract and appropriate immunologic response to these non-self antigens is essential to the survival of the species. In light of the weight of this task, it is not surprising that multiple, redundant and overlapping mechanisms are involved. For instance, cells at the immunologic interface between self (female reproductive tract epithelium) and non-self (placental trophoblast cells or human sperm) express glycosylation patterns that mimic those on many metastatic cancer cells and successful pathogens. The cytokine/chemokine milieu at this interface is altered through endocrine and immunologic mechanisms to favor tolerance of non-self. The "foreign" cells themselves also play an integral role in their own immunologic acceptance, since sperm and placental trophoblast cells are unusual and unique in their antigen presenting molecule expression patterns. Here, we will discuss these and other mechanisms that allow the human female reproductive tract to perform this delicate and indispensible balancing act.
Keywords: cervix; human; immune privilege; semen; trophoblast; vagina.
Figures
References
-
- Abe K., McKibbin J. M., Hakomori S. (1983). The monoclonal antibody directed to difucosylated type 2 chain (Fucα1-2Galβ1-4[Fucα1-3]GlcNAc; Y Determinant). J. Biol. Chem. 258, 11793–11797 - PubMed
-
- Adachi M., Hayami M., Kashiwagi N., Mizuta T., Ohta Y., Gill M. J., et al. (1988). Expression of Ley antigen in human immunodeficiency virus-infected human T cell lines and in peripheral lymphocytes of patients with acquired immune deficiency syndrome (AIDS) and AIDS-related complex (ARC). J. Exp. Med. 167, 323–331 - PMC - PubMed
-
- Allen R. D., Roberts T. K. (1986). The relationship between the immunosuppressive and cytotoxic effects of human seminal plasma. Am. J. Reprod. Immunol. Microbiol. 11, 59–64 - PubMed
-
- Allen R. D., Roberts T. K. (1987). Role of spermine in the cytotoxic effects of seminal plasma. Am. J. Reprod. Immunol. Microbiol. 13, 4–8 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

