Non-invasive imaging for studying anti-angiogenic therapy effects
- PMID: 23407722
- PMCID: PMC3810587
- DOI: 10.1160/TH12-10-0721
Non-invasive imaging for studying anti-angiogenic therapy effects
Abstract
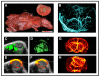
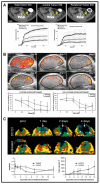
Noninvasive imaging plays an emerging role in preclinical and clinical cancer research and has high potential to improve clinical translation of new drugs. This article summarises and discusses tools and methods to image tumour angiogenesis and monitor anti-angiogenic therapy effects. In this context, micro-computed tomography (µCT) is recommended to visualise and quantify the micro-architecture of functional tumour vessels. Contrast-enhanced ultrasound (US) and magnetic resonance imaging (MRI) are favourable tools to assess functional vascular parameters, such as perfusion and relative blood volume. These functional parameters have been shown to indicate anti-angiogenic therapy response at an early stage, before changes in tumour size appear. For tumour characterisation, the imaging of the molecular characteristics of tumour blood vessels, such as receptor expression, might have an even higher diagnostic potential and has been shown to be highly suitable for therapy monitoring as well. In this context, US using targeted microbubbles is currently evaluated in clinical trials as an important tool for the molecular characterisation of the angiogenic endothelium. Other modalities, being preferably used for molecular imaging of vessels and their surrounding stroma, are photoacoustic imaging (PAI), near-infrared fluorescence optical imaging (OI), MRI, positron emission tomography (PET) and single photon emission computed tomography (SPECT). The latter two are particularly useful if very high sensitivity is needed, and/or if the molecular target is difficult to access. Carefully considering the pros and cons of different imaging modalities in a multimodal imaging setup enables a comprehensive longitudinal assessment of the (micro)morphology, function and molecular regulation of tumour vessels.
Figures

References
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. - PubMed
-
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

