DNA immunization as an efficient strategy for vaccination
- PMID: 23407787
- PMCID: PMC3558129
DNA immunization as an efficient strategy for vaccination
Abstract
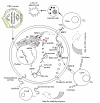
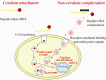
The field of vaccinology provides excellent promises to control different infectious and non-infectious diseases. Genetic immunization as a new tool in this area by using naked DNA has been shown to induce humoral as well as cellular immune responses with high efficiency. This demonstrates the enormous potential of this strategy for vaccination purposes. DNA vaccines have been widely used to develop vaccines against various pathogens as well as cancer, autoimmune diseases and allergy. However, despite their successful application in many pre-clinical disease models, their potency in human clinical trials has been insufficient to provide protective immunity. Several strategies have been applied to increase the potency of DNA vaccine. Among these strategies, the linkage of antigens to Heat Shock Proteins (HSPs) and the utilization of different delivery systems have been demonstrated as efficient approaches for increasing the potency of DNA vaccines. The uptake of DNA plasmids by cells upon injection is inefficient. Two basic delivery approaches including physical delivery to achieve higher levels of antigen production and formulation with microparticles to target Antigen-Presenting Cells (APCs) are effective in animal models. Alternatively, different regimens called prime-boost vaccination are also effective. In this regimen, naked DNA is utilized to prime the immune system and either recombinant viral vector or purified recombinant protein with proper adjuvant is used for boosting. In this review, we discuss recent advances in upgrading the efficiency of DNA vaccination in animal models.
Keywords: Adjuvant; DNA vaccination; Delivery system; Infectious disease; Prime-boost vaccination.
Figures
Similar articles
-
Prime-boost vaccine strategy against viral infections: Mechanisms and benefits.Vaccine. 2016 Jan 20;34(4):413-423. doi: 10.1016/j.vaccine.2015.11.062. Epub 2015 Dec 13. Vaccine. 2016. PMID: 26691569 Review.
-
Cross-Protection against MERS-CoV by Prime-Boost Vaccination Using Viral Spike DNA and Protein.J Virol. 2020 Nov 23;94(24):e01176-20. doi: 10.1128/JVI.01176-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32967955 Free PMC article.
-
Enhanced immune responses to E2 protein and DNA formulated with ISA 61 VG administered as a DNA prime-protein boost regimen against bovine viral diarrhea virus.Vaccine. 2018 Sep 5;36(37):5591-5599. doi: 10.1016/j.vaccine.2018.07.054. Epub 2018 Aug 1. Vaccine. 2018. PMID: 30077483
-
Prime/boost immunization with HIV-1 MPER-V3 fusion construct enhances humoral and cellular immune responses.Immunol Lett. 2015 Dec;168(2):366-73. doi: 10.1016/j.imlet.2015.10.012. Epub 2015 Oct 27. Immunol Lett. 2015. PMID: 26518142
-
Prime-boost immunization strategies for infectious diseases.Curr Opin Mol Ther. 2002 Feb;4(1):23-7. Curr Opin Mol Ther. 2002. PMID: 11883691 Review.
Cited by
-
Innovative Immunization Strategies for Antivenom Development.Toxins (Basel). 2018 Nov 2;10(11):452. doi: 10.3390/toxins10110452. Toxins (Basel). 2018. PMID: 30400220 Free PMC article. Review.
-
Induction and maintenance of bi-functional (IFN-γ + IL-2+ and IL-2+ TNF-α+) T cell responses by DNA prime MVA boosted subtype C prophylactic vaccine tested in a Phase I trial in India.PLoS One. 2019 Mar 28;14(3):e0213911. doi: 10.1371/journal.pone.0213911. eCollection 2019. PLoS One. 2019. PMID: 30921340 Free PMC article. Clinical Trial.
-
Persistence of Hepatitis B Virus Infection: A Multi-Faceted Player for Hepatocarcinogenesis.Front Microbiol. 2021 Aug 30;12:678537. doi: 10.3389/fmicb.2021.678537. eCollection 2021. Front Microbiol. 2021. PMID: 34526974 Free PMC article. Review.
-
Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development.Arch Med Res. 2021 Jan;52(1):15-24. doi: 10.1016/j.arcmed.2020.09.010. Epub 2020 Sep 10. Arch Med Res. 2021. PMID: 32950264 Free PMC article. Review.
-
Mini-chaperones: potential immuno-stimulators in vaccine design.Hum Vaccin Immunother. 2013 Jan;9(1):153-61. doi: 10.4161/hv.22248. Epub 2012 Oct 29. Hum Vaccin Immunother. 2013. PMID: 23108356 Free PMC article. Review.
References
-
- Ertl PF, Thomsen LL. Technical issues in construction of nucleic acid vaccines. Methods. 2003;31(3):199–206. - PubMed
-
- Sharma AK, Khuller GK. DNA vaccines: future strategies and relevance to intracellular pathogens: A review. Immunol Cell Biol. 2001;79(6):537–546. - PubMed
-
- Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60(4):1035–1042. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
