Cloning and Expression of Leishmania infantum LPG3 Gene by the Lizard Leishmania Expression System
- PMID: 23407850
- PMCID: PMC3558223
Cloning and Expression of Leishmania infantum LPG3 Gene by the Lizard Leishmania Expression System
Abstract
Background: Various prokaryotic and eukaryotic expression systems have been developed for the production of recombinant proteins. In the present study, we used a new protein expression system based on the Iranian Lizard Leishmania, a trypanosomatid protozoan as a host, for the expression of LPG3 gene from Leishmania infantum (L.infantum).
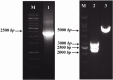
Methods: The LPG3 gene was cloned in the expression cassette for integration into the small subunit of the ribosomal RNA locus of Lizard Leishmania genome by electroporation. Expression of the recombinant LPG3 protein was confirmed by western blotting and immunofluorescence staining.
Results: Western blotting confirmed the expression and production of rLPG3 protein. Immunofluoresence analysis also revealed the staining throughout the cytoplasm of transfected parasites, indicating that the protein has been expressed.
Conclusion: These results demonstrate that Leishmania cells can be suggested an expression system for the production of recombinant LPG3 (rLPG3) to further research in vaccine designing against leishmaniasis.
Keywords: Leishmania; Leishmania infantum; Recombinant proteins; Vaccines.
Figures




Similar articles
-
Lipophosphoglycan-3 protein from Leishmania infantum chagasi plus saponin adjuvant: A new promising vaccine against visceral leishmaniasis.Vaccine. 2021 Jan 8;39(2):282-291. doi: 10.1016/j.vaccine.2020.11.064. Epub 2020 Dec 11. Vaccine. 2021. PMID: 33309484
-
Lipophosphoglycan-3 recombinant protein vaccine controls hepatic parasitism and prevents tissue damage in mice infected by Leishmania infantum chagasi.Biomed Pharmacother. 2020 Jun;126:110097. doi: 10.1016/j.biopha.2020.110097. Epub 2020 Mar 20. Biomed Pharmacother. 2020. PMID: 32203891
-
Immune response to recombinant Leishmania infantum lipophosphoglycan 3 plus CpG oligodeoxynucleotides in BALB/c mice.Parasite Immunol. 2017 Mar;39(3). doi: 10.1111/pim.12345. Parasite Immunol. 2017. PMID: 27353355
-
A Non-pathogenic Recombinant Leishmania Expressing Lipophosphoglycan 3 Against Experimental Infection with Leishmania infantum.Scand J Immunol. 2017 Jul;86(1):15-22. doi: 10.1111/sji.12557. Scand J Immunol. 2017. PMID: 28426153
-
Immune response in susceptible BALB/c mice immunized with DNA encoding Lipophosphoglycan 3 of Leishmania infantum.Parasite Immunol. 2014 Dec;36(12):700-7. doi: 10.1111/pim.12147. Parasite Immunol. 2014. PMID: 25244583
Cited by
-
Immunogenicity evaluation of a rationally designed polytope construct encoding HLA-A*0201 restricted epitopes derived from Leishmania major related proteins in HLA-A2/DR1 transgenic mice: steps toward polytope vaccine.PLoS One. 2014 Oct 13;9(10):e108848. doi: 10.1371/journal.pone.0108848. eCollection 2014. PLoS One. 2014. PMID: 25310094 Free PMC article.
References
-
- Basile G, Peticca M. Recombinant protein expression in Leishmania tarentolae. Mol Biotechnol. 2009;43(3):273–278. - PubMed
-
- Nagill R, Kuar S. Vaccine candidates for leish-maniasis: A review. Int Immunopharm. 2011;11(10):1464–1488. - PubMed
-
- Goyal N, Duncan R, Selvapandiyan A, Debrabant A, Baig MS, Nakhasi HL. Cloning and characterization of angiotension converting enzyme related dipeptidylcarboxypeptidase from Leishmania donovani. Mol Biochem Parasitol. 2006;145(2):147–157. - PubMed
-
- Ilg T, Demar M, Harbecke D. Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J Biol Chem. 2001;276(7):4988–4997. - PubMed
LinkOut - more resources
Full Text Sources
