A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia
- PMID: 23407943
- PMCID: PMC3711390
- DOI: 10.1523/JNEUROSCI.1943-12.2013
A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia
Abstract
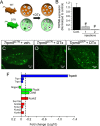
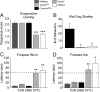
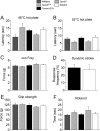
Many primary sensory neurons are polymodal, responding to multiple stimulus modalities (chemical, thermal, or mechanical), yet each modality is recognized differently. Although polymodality implies that stimulus encoding occurs in higher centers, such as the spinal cord or brain, recent sensory neuron ablation studies find that behavioral responses to different modalities require distinct subpopulations, suggesting the existence of modality-specific labeled lines at the level of the sensory afferent. Here we provide evidence that neurons expressing TRPM8, a cold- and menthol-gated channel required for normal cold responses in mammals, represents a labeled line solely for cold sensation. We examined the behavioral significance of conditionally ablating TRPM8-expressing neurons in adult mice, finding that, like animals lacking TRPM8 channels (Trpm8(-/-)), animals depleted of TRPM8 neurons ("ablated") are insensitive to cool to painfully cold temperatures. Ablated animals showed little aversion to noxious cold and did not distinguish between cold and a preferred warm temperature, a phenotype more profound than that of Trpm8(-/-) mice which exhibit only partial cold-avoidance and -preference behaviors. In addition to acute responses, cold pain associated with inflammation and nerve injury was significantly attenuated in ablated and Trpm8(-/-) mice. Moreover, cooling-induced analgesia after nerve injury was abolished in both genotypes. Last, heat, mechanical, and proprioceptive behaviors were normal in ablated mice, demonstrating that TRPM8 neurons are dispensable for other somatosensory modalities. Together, these data show that, although some limited cold sensitivity remains in Trpm8(-/-) mice, TRPM8 neurons are required for the breadth of behavioral responses evoked by cold temperatures.
Figures





References
-
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
