Preventing formation of reticulon 3 immunoreactive dystrophic neurites improves cognitive function in mice
- PMID: 23407961
- PMCID: PMC3711383
- DOI: 10.1523/JNEUROSCI.2445-12.2013
Preventing formation of reticulon 3 immunoreactive dystrophic neurites improves cognitive function in mice
Abstract
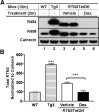
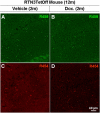
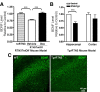
Neuritic dystrophy is one of the important pathological features associated with amyloid plaques in Alzheimer's disease (AD) and age-dependent neuronal dysfunctions. We reported previously that reticulon-3 (RTN3) immunoreactive dystrophic neurites (RIDNs) are abundantly present in the hippocampus of AD patients, in AD mouse models, and in aged wild-type mice. Transgenic mice overexpressing the human RTN3 transgene spontaneously develop RIDNs in their hippocampi, and the formation of RIDNs correlates with the appearance of RTN3 aggregation. To further elucidate whether the formation of RIDNs is reversible, we generated transgenic mice expressing wild-type human RTN3 under the control of a tetracycline-responsive promoter. Treatment with doxycycline for 2 months effectively turned off expression of the human RTN3 transgene, confirming the inducible nature of the system. However, the formation of hippocampal RIDNs was dependent on whether the transgene was turned off before or after the formation of RTN3 aggregates. When transgenic human RTN3 expression was turned off at young age, formation of RIDNs was essentially eliminated compared with the vehicle-treated transgenic mice. More importantly, a fear conditioning study demonstrated that contextual associative learning and memory in inducible transgenic mice was improved if the density of RIDNs was lowered. Additional mechanistic study suggested that a reduction in BDNF levels in transgenic mice might contribute to the reduced learning and memory in transgenic mice overexpressing RTN3. Hence, we conclude that age-dependent RIDNs cannot be effectively cleared once they have formed, and we postulate that successful prevention of RIDN formation should be initiated before RTN3 aggregation.
Figures





Similar articles
-
Reduced amyloid deposition in mice overexpressing RTN3 is adversely affected by preformed dystrophic neurites.J Neurosci. 2009 Jul 22;29(29):9163-73. doi: 10.1523/JNEUROSCI.5741-08.2009. J Neurosci. 2009. PMID: 19625507 Free PMC article.
-
Transgenic mice overexpressing reticulon 3 develop neuritic abnormalities.EMBO J. 2007 Jun 6;26(11):2755-67. doi: 10.1038/sj.emboj.7601707. Epub 2007 May 3. EMBO J. 2007. PMID: 17476306 Free PMC article.
-
The occurrence of aging-dependent reticulon 3 immunoreactive dystrophic neurites decreases cognitive function.J Neurosci. 2009 Apr 22;29(16):5108-15. doi: 10.1523/JNEUROSCI.5887-08.2009. J Neurosci. 2009. PMID: 19386906 Free PMC article.
-
Effects of altered RTN3 expression on BACE1 activity and Alzheimer's neuritic plaques.Rev Neurosci. 2017 Feb 1;28(2):145-154. doi: 10.1515/revneuro-2016-0054. Rev Neurosci. 2017. PMID: 27883331 Review.
-
Transgenic mouse models of Alzheimer's disease.Ann N Y Acad Sci. 2000 Jun;908:260-6. doi: 10.1111/j.1749-6632.2000.tb06653.x. Ann N Y Acad Sci. 2000. PMID: 10911965 Review.
Cited by
-
Neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma.Invest Ophthalmol Vis Sci. 2015 Jan 13;56(2):893-907. doi: 10.1167/iovs.14-15008. Invest Ophthalmol Vis Sci. 2015. PMID: 25587060 Free PMC article.
-
Inhibiting BACE1 to reverse synaptic dysfunctions in Alzheimer's disease.Neurosci Biobehav Rev. 2016 Jun;65:326-40. doi: 10.1016/j.neubiorev.2016.03.025. Epub 2016 Apr 1. Neurosci Biobehav Rev. 2016. PMID: 27044452 Free PMC article. Review.
-
A New Vision for Therapeutic Hypothermia in the Era of Targeted Temperature Management: A Speculative Synthesis.Ther Hypothermia Temp Manag. 2019 Mar;9(1):13-47. doi: 10.1089/ther.2019.0001. Epub 2019 Feb 25. Ther Hypothermia Temp Manag. 2019. PMID: 30802174 Free PMC article. Review.
-
Impact of RTN3 deficiency on expression of BACE1 and amyloid deposition.J Neurosci. 2014 Oct 15;34(42):13954-62. doi: 10.1523/JNEUROSCI.1588-14.2014. J Neurosci. 2014. PMID: 25319692 Free PMC article.
-
Dynactin 6 deficiency enhances aging-associated dystrophic neurite formation in mouse brains.Neurobiol Aging. 2021 Nov;107:21-29. doi: 10.1016/j.neurobiolaging.2021.07.006. Epub 2021 Jul 15. Neurobiol Aging. 2021. PMID: 34371284 Free PMC article.
References
-
- Araki W, Oda A, Motoki K, Hattori K, Itoh M, Yuasa S, Konishi Y, Shin RW, Tamaoka A, Ogino K. Reduction of β-amyloid accumulation by reticulon 3 in transgenic mice. Curr Alzheimer Res. 2013 in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
