Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice
- PMID: 23407970
- PMCID: PMC3596815
- DOI: 10.1523/JNEUROSCI.1046-12.2013
Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice
Abstract
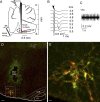
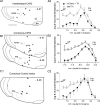
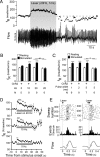
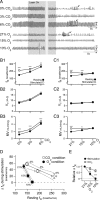
Activation of rostral ventrolateral medullary catecholaminergic (RVLM-CA) neurons e.g., by hypoxia is thought to increase sympathetic outflow thereby raising blood pressure (BP). Here we test whether these neurons also regulate breathing and cardiovascular variables other than BP. Selective expression of ChR2-mCherry by RVLM-CA neurons was achieved by injecting Cre-dependent vector AAV2-EF1α-DIO-ChR2-mCherry unilaterally into the brainstem of dopamine-β-hydroxylase(Cre/0) mice. Photostimulation of RVLM-CA neurons increased breathing in anesthetized and conscious mice. In conscious mice, photostimulation primarily increased breathing frequency and this effect was fully occluded by hypoxia (10% O(2)). In contrast, the effects of photostimulation were largely unaffected by hypercapnia (3 and 6% CO(2)). The associated cardiovascular effects were complex (slight bradycardia and hypotension) and, using selective autonomic blockers, could be explained by coactivation of the sympathetic and cardiovagal outflows. ChR2-positive RVLM-CA neurons expressed VGLUT2 and their projections were mapped. Their complex cardiorespiratory effects are presumably mediated by their extensive projections to supraspinal sites such as the ventrolateral medulla, the dorsal vagal complex, the dorsolateral pons, and selected hypothalamic nuclei (dorsomedial, lateral, and paraventricular nuclei). In sum, selective optogenetic activation of RVLM-CA neurons in conscious mice revealed two important novel functions of these neurons, namely breathing stimulation and cardiovagal outflow control, effects that are attenuated or absent under anesthesia and are presumably mediated by the numerous supraspinal projections of these neurons. The results also suggest that RVLM-CA neurons may underlie some of the acute respiratory response elicited by carotid body stimulation but contribute little to the central respiratory chemoreflex.
Figures


References
-
- Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. - PubMed
-
- Arango V, Ruggiero DA, Callaway JL, Anwar M, Mann JJ, Reis DJ. Catecholaminergic neurons in the ventrolateral medulla and nucleus of the solitary tract in the human. J Comp Neurol. 1988;273:224–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
