Gastrin-induced proliferation involves MEK partner 1 (MP1)
- PMID: 23408059
- PMCID: PMC3611038
- DOI: 10.1007/s11626-013-9588-2
Gastrin-induced proliferation involves MEK partner 1 (MP1)
Abstract
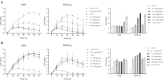
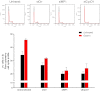
The peptide hormone gastrin is an important factor for the maintenance and homeostasis of the gastric mucosa. We show that gastrin stimulates proliferation in a dose-dependent manner in the human gastric adenocarcinoma cell line AGS-GR. Furthermore, we demonstrate that the MAPK scaffold protein MEK partner 1 (MP1) is important for gastrin-induced phosphorylation of ERK1 and ERK2 and that MP1 promotes gastrin-induced proliferation of AGS-GR cells. Our results suggest a role of MP1 in gastrin-induced cellular responses involved in proliferation and homeostasis of the gastric mucosa.
Figures


Similar articles
-
Regulation of protein phosphorylation within the MKK1-ERK2 complex by MP1 and the MP1*P14 heterodimer.Arch Biochem Biophys. 2007 Apr 1;460(1):85-91. doi: 10.1016/j.abb.2006.11.031. Epub 2007 Jan 4. Arch Biochem Biophys. 2007. PMID: 17254543 Free PMC article.
-
BCL2 induced by LAMTOR3/MAPK is a druggable target of chemoradioresistance in mesenchymal lung cancer.Cancer Lett. 2017 Sep 10;403:48-58. doi: 10.1016/j.canlet.2017.05.019. Epub 2017 Jun 10. Cancer Lett. 2017. PMID: 28606806
-
Gastrin stimulates MMP-1 expression in gastric epithelial cells: putative role in gastric epithelial cell migration.Am J Physiol Gastrointest Liver Physiol. 2015 Jul 15;309(2):G78-86. doi: 10.1152/ajpgi.00084.2015. Epub 2015 May 14. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25977510 Free PMC article.
-
MEK partner 1 (MP1): regulation of oligomerization in MAP kinase signaling.J Cell Biochem. 2005 Mar 1;94(4):708-19. doi: 10.1002/jcb.20344. J Cell Biochem. 2005. PMID: 15547943
-
ERK1/2 MAP kinases: structure, function, and regulation.Pharmacol Res. 2012 Aug;66(2):105-43. doi: 10.1016/j.phrs.2012.04.005. Epub 2012 Apr 27. Pharmacol Res. 2012. PMID: 22569528 Review.
Cited by
-
The duration of gastrin treatment affects global gene expression and molecular responses involved in ER stress and anti-apoptosis.BMC Genomics. 2013 Jun 28;14:429. doi: 10.1186/1471-2164-14-429. BMC Genomics. 2013. PMID: 23805861 Free PMC article.
-
Optimization of in vitro trophoblast assay for real-time impedimetric sensing of trophoblast-erythrocyte interactions in Plasmodium falciparum malaria.Anal Bioanal Chem. 2020 Jun;412(16):3915-3923. doi: 10.1007/s00216-020-02413-1. Epub 2020 Jan 27. Anal Bioanal Chem. 2020. PMID: 31989195 Free PMC article.
-
Salt-inducible kinase 1 (SIK1) is induced by gastrin and inhibits migration of gastric adenocarcinoma cells.PLoS One. 2014 Nov 10;9(11):e112485. doi: 10.1371/journal.pone.0112485. eCollection 2014. PLoS One. 2014. PMID: 25384047 Free PMC article.
-
Clinical-grade human dental pulp stem cells suppressed the activation of osteoarthritic macrophages and attenuated cartilaginous damage in a rabbit osteoarthritis model.Stem Cell Res Ther. 2021 May 1;12(1):260. doi: 10.1186/s13287-021-02353-2. Stem Cell Res Ther. 2021. PMID: 33933140 Free PMC article.
-
Gastrin Enhances Autophagy and Promotes Gastric Carcinoma Proliferation via Inducing AMPKα.Oncol Res. 2017 Sep 21;25(8):1399-1407. doi: 10.3727/096504016X14823648620870. Epub 2017 Jan 5. Oncol Res. 2017. PMID: 28059052 Free PMC article.
References
-
- Detjen K, Yule D, Tseng MJ, Williams JA, Logsdon CD. CCK-B receptors produce similar signals but have opposite growth effects in CHO and Swiss 3T3 cells. Am J Physiol. 1997b;273:C1449–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

