Regional cortical and trabecular bone loss after spinal cord injury
- PMID: 23408218
- PMCID: PMC3647247
- DOI: 10.1682/jrrd.2011.12.0245
Regional cortical and trabecular bone loss after spinal cord injury
Abstract
Spinal cord injury (SCI) triggers rapid loss of trabecular bone mineral density (BMD) in bone epiphyses and a loss of cortical cross-sectional area (CSA) in bone diaphyses, increasing fracture risk for people with SCI. The purpose of this study was to measure trabecular BMD and cortical CSA loss at several previously unexamined lower-limb sites (4% fibula, 12% femur, 86% tibia, cortical) in individuals with SCI. Using peripheral quantitative computed tomography, we scanned 13 participants with SCI longitudinally and 16 on one occasion; 21 participants without SCI served as controls. In the first year post-SCI, 15% to 35% of BMD was lost at the distal femur, proximal tibia, and distal fibula. Bone loss at the distal fibula accelerated between 1 and 2 years post-SCI. BMD at these sites reached a steady state value of ~50% of the non-SCI value 4 years post-SCI. At the tibia diaphysis, cortical CSA decline was slower, eventually reaching 65% of the non-SCI value. Because of the extensive loss of bone observed at these sites, careful consideration needs to be given to the dose of musculoskeletal stress delivered during rehabilitation interventions like standing, muscle electrical stimulation, and aggressive stretching of spastic muscles.
Figures

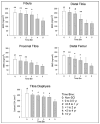
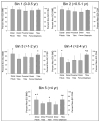

References
-
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–60. http://dx.doi.org/10.1016/0021-9290(94)90010-8. - DOI - PubMed
-
- Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292(1):C545–52. http://dx.doi.org/10.1152/ajpcell.00611.2005. - DOI - PubMed
-
- Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13(9):688–700. http://dx.doi.org/10.1007/s001980200095. - DOI - PubMed
-
- Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30(3):445–52. http://dx.doi.org/10.1016/S8756-3282(01)00689-5. - DOI - PubMed
-
- Heinonen A, Sievänen H, Kannus P, Oja P, Vuori I. Site-specific skeletal response to long-term weight training seems to be attributable to principal loading modality: a pQCT study of female weightlifters. Calcif Tissue Int. 2002;70(6):469–74. http://dx.doi.org/10.1007/s00223-001-1019-9. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
