Control of breathing by interacting pontine and pulmonary feedback loops
- PMID: 23408512
- PMCID: PMC3570896
- DOI: 10.3389/fncir.2013.00016
Control of breathing by interacting pontine and pulmonary feedback loops
Abstract
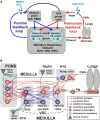
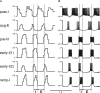
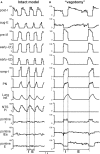
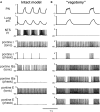
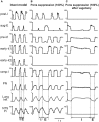
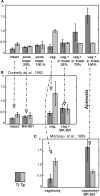
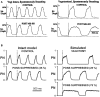
The medullary respiratory network generates respiratory rhythm via sequential phase switching, which in turn is controlled by multiple feedbacks including those from the pons and nucleus tractus solitarii; the latter mediates pulmonary afferent feedback to the medullary circuits. It is hypothesized that both pontine and pulmonary feedback pathways operate via activation of medullary respiratory neurons that are critically involved in phase switching. Moreover, the pontine and pulmonary control loops interact, so that pulmonary afferents control the gain of pontine influence of the respiratory pattern. We used an established computational model of the respiratory network (Smith et al., 2007) and extended it by incorporating pontine circuits and pulmonary feedback. In the extended model, the pontine neurons receive phasic excitatory activation from, and provide feedback to, medullary respiratory neurons responsible for the onset and termination of inspiration. The model was used to study the effects of: (1) "vagotomy" (removal of pulmonary feedback), (2) suppression of pontine activity attenuating pontine feedback, and (3) these perturbations applied together on the respiratory pattern and durations of inspiration (T(I)) and expiration (T(E)). In our model: (a) the simulated vagotomy resulted in increases of both T(I) and T(E), (b) the suppression of pontine-medullary interactions led to the prolongation of T(I) at relatively constant, but variable T(E), and (c) these perturbations applied together resulted in "apneusis," characterized by a significantly prolonged T(I). The results of modeling were compared with, and provided a reasonable explanation for, multiple experimental data. The characteristic changes in T(I) and T(E) demonstrated with the model may represent characteristic changes in the balance between the pontine and pulmonary feedback control mechanisms that may reflect specific cardio-respiratory disorders and diseases.
Keywords: apneusis; brainstem; control of breathing; pontine-medullary interactions; pre-Bötzinger complex; pulmonary feedback; respiratory central pattern generator; ventrolateral respiratory column.
Figures
References
-
- Anders K., Ohndorf W., Dermietzel R., Richter D. W. (1993). Synapses between slowly adapting lung stretch receptor afferents and inspiratory beta-neurons in the nucleus of the solitary tract of cats: a light and electron microscopic analysis. J. Comp. Neurol. 335, 163–172 10.1002/cne.903350203 - DOI - PubMed
-
- Averill D. B., Cameron W. E., Berger A. J. (1984). Monosynaptic excitation of dorsal medullary respiratory neurons by slowly adapting pulmonary stretch receptors. J. Neurophysiol. 52, 771–785 - PubMed
-
- Backman S. B., Anders C., Ballantyne D., Rohrig N., Camerer H., Mifflin S., et al. (1984). Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflugers Arch. 402, 129–136 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

